Bio-Rad has received AFNOR validation for the iQ-Check Legionella Aquadien DNA extraction short protocols including the addition of the Free DNA Removal Solution protocol according to ISO TS 12869 and NF T90-471.
These validations allow Bio-Rad to be the first and only Legionella PCR method validated by a third party that includes management of free DNA in the sample.
Bio-Rad provides a complete range of products for the detection and quantification of Legionella. Products comply with regulatory standards and allow for risk management based on accurate and rapid results. These Legionella testing products are suitable for water supply systems, water intended for human consumption, hot water for sanitation purposes, water for industrial use, natural mineral water, water for recreational activities, biofilms, and aerosol samples.
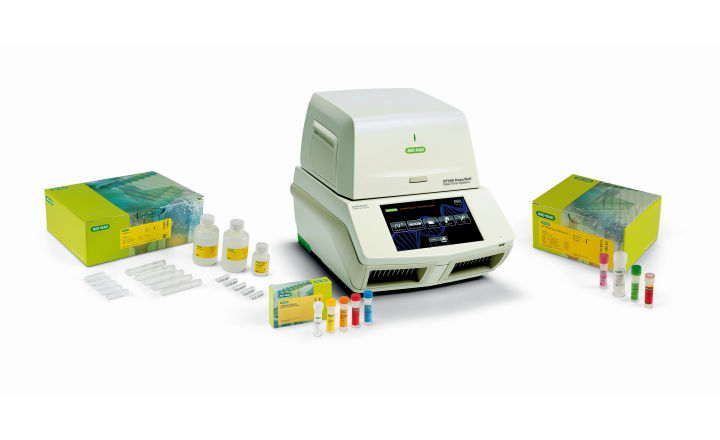
PCR range includes iQ-Check Legionella spp. and L. pneumophilia kits, associated with the high-performance Aquadien DNA Extraction kit
- Compatible with all types and volume of water (water systems, drinking water as well as cooling tower water)
- Results delivered in 4 hours from sample receipt with no confirmation needed
- Can detect and quantify Legionella spp. and L. pneumophilia in the same assay
- 96 well format allows for an adaptable number of assays per run
- Fully automated results with internal positive and negative control
- Free DNA Removal Solution option to improve the reliability of results
- AFNOR VALIDATION certified according to NF T90-471 and ISO TS 12869
- Reliable for colony confirmation according to NF T90-431 and NF EN ISO 11131
Watch Bio-Rad's Recorded Webinar on Legionella Testing Solutions and learn more at bio-rad.com/water
Important information from the European Parliament:
To learn more click on the green "Request Information" button below.