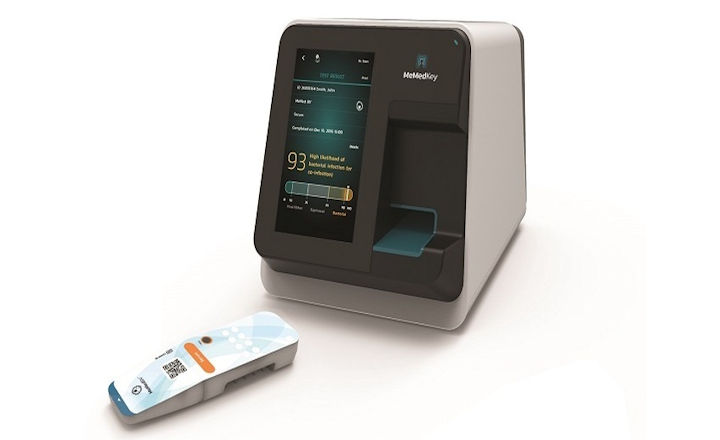
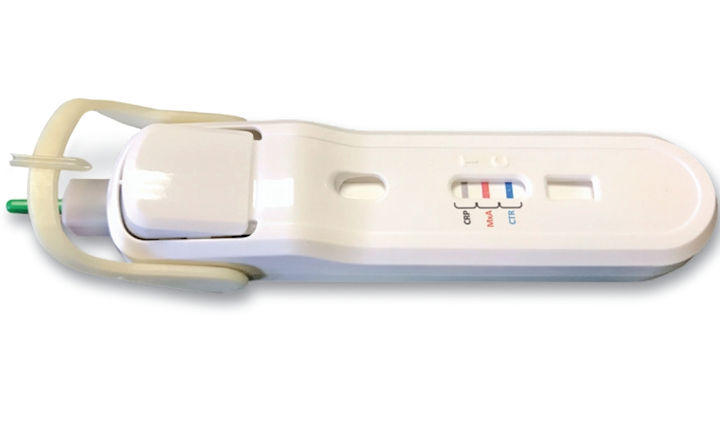
MeMed has announced its MeMed BV® test has received 510(k) clearance from the US FDA. The test is used to help healthcare providers distinguish between bacterial and viral infections. The technology has been cleared for both children and adults. The test is for point-of-care at runs on the MeMed Key® platform.
Bacterial and viral infections are often clinically indistinguishable, leading to the prescription of antibiotics for the treatment of viral infections, for which they are ineffective. Antibiotic misuse drives the emergence of antimicrobial resistance (AMR), one of the biggest healthcare challenges of our time.
The novelty of MeMed’s technology is that it decodes the body’s immune response to infection, the ‘host response’, rather than focusing on detecting the presence of a microbe. This allows robust diagnosis when the infection site is inaccessible or unknown, even when the pathogen is undetectable using conventional tests, or when the cause of infection is emerging new pathogens. It enables better informed antibiotic treatment decisions, an essential tool in the fight against resistant bacteria.
MeMed BV® measures and computationally integrates the levels of three immune system proteins: TRAIL, IP-10, and CRP. When run on the MeMed Key® platform, MeMed BV® provides a result within 15 minutes.
The test provides highly accurate results with Area Under the Curve of 90% and 97% (primary and secondary endpoints). MeMed has established its US base in Boston and is ramping up commercial activities to ensure broad availability of its products across the US.
FDA clearance was based on a multi-center blinded clinical validation study enrolling over 1,000 children and adults and addresses goals laid out in the US National Action Plan for Combating Antibiotic-Resistant Bacteria.
Request information using button provided below or visit memed.com to learn more.
Note: This content has been edited by a rapidmicrobiology staff writer for style and content.