3M Food Safety have results of two new scientific studies conducted by Cardiff Metropolitan University in Wales, U.K. and commissioned by 3M. The research shows significant differences between the performance of seven brands of ATP hygiene monitoring systems when evaluating the impact of time and temperature on the accuracy and repeatability of test results.
All seven systems test for the presence of Adenosine Triphosphate (ATP), an indicator of biological residue post cleaning, that provides an overall measure of cleanliness in the food processing environment, test results are used to help manufacturers make the high-risk decision to start food manufacturing and confidently manage risk. The research underscores that not all systems are the same, and that it is critical to select and use methods that consistently produce accurate and reliable results.
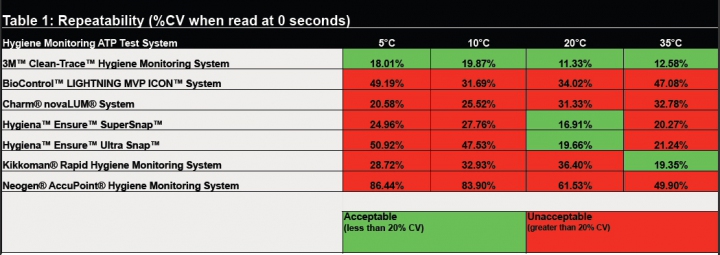
The first study, “Evaluation of Repeatability of Surface ATP Test Devices at Different Environmental Temperatures,” examined the reliability of testing devices to accurately and consistently detect a known amount of ATP. Because food manufacturers operate in a wide range of temperatures, tests were conducted at four temperatures. Systems were evaluated at 5°C, 10°C, 20°C and 35°C. To test repeatability of results, a known amount of ATP was measured and read. System variation was expressed as coefficient of variation (CV), where the larger the percent CV, the greater the risk of inaccurate and unreliable results. A CV greater than 20 percent was considered unacceptable. In this study, the 3M™ Clean-Trace™ Hygiene Monitoring System provided the most consistent and repeatable readings. It was the only hygiene monitoring system to consistently achieve less than 20 percent CV at all temperatures. In fact, three systems produced unacceptable results at all temperatures and the other three produced inconsistent results. (Table 1).
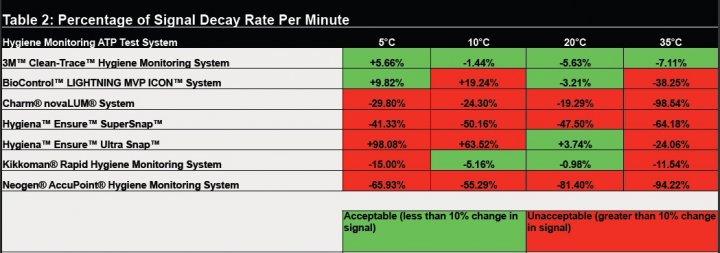
The second study, “Evaluation of Time Dependency of Surface ATP Test Devices at Different Environmental Temperatures” takes the review process a step further. The time it takes to conduct a test can differ when testing the same site from day to day or even between shifts due to interruptions during testing or differences in technique of various technicians. This study evaluated the impact of time on stability and accuracy of results generated by the seven hygiene monitoring systems. To test accuracy of results, a known amount of ATP was measured and read at 20- second intervals over two minutes to determine how much results varied. System variation was expressed as percent signal decay per minute. A signal decay of more than 10 percent per minute was considered unacceptable. Systems were again evaluated at 5°C, 10°C, 20°C and 35°C. In this study, three of the systems produced unacceptable results at all levels while the other three produced inconsistent results. The 3M Clean-Trace Hygiene Monitoring System was the only system that produced acceptable results at all temperatures. (Table 2)
These studies demonstrate that all hygiene monitoring systems do not provide the same quality of results. The only system that was stable and repeatable across both time and temperature was the 3M Clean-Trace Hygiene Monitoring System. All other systems had unacceptable results in one or both tests.
In their conclusions, the Cardiff University scientists cautioned that the use of systems that are “highly time or temperature dependent or had poor repeatability could lead to highly inaccurate and unreliable results.” They also stated that “it is of paramount importance that hygiene monitoring systems provide a repeatable result to ensure consistency and accuracy of results.”
As a leader of innovative solutions that help the food and beverage industries optimize the quality and safety of their products to enable consumer protection, 3M Food Safety commissioned this research in 2014.