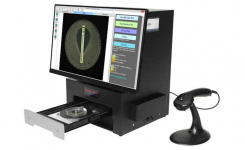
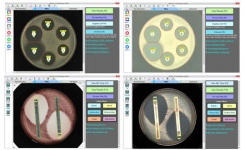
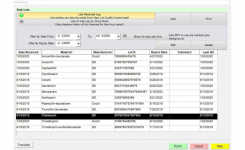
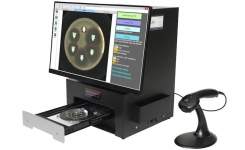
BIOMIC V3 (Giles Scientific Inc, California USA) is a digital imaging system for automating the reading and interpretation of AST and ID tests from various manufacturers. The following guidelines are updated in the 2022 BIOMIC V3 software:
EUCAST Guidelines:
- Breakpoint Tables for Interpretation of MICs and Zone Diameters. v 12.0 (January 2022)
- Routine and extended internal quality control for MIC determination and disk diffusion. v 12.0 (January 2022)
- Zone diameter breakpoints for rapid antimicrobial susceptibility testing (RAST) directly from blood culture bottles. v 5.0 (April 2022)
CLSI Guidelines:
- M100, 32nd edition: Performance Standards for Antimicrobial Susceptibility Testing (January 2022)
Visit www.biomic.com to learn more or click on the Request Information button below.
Please note : Any products described on this page are
for Research Use Only and not intended for clinical diagnostic procedures unless otherwise stated.