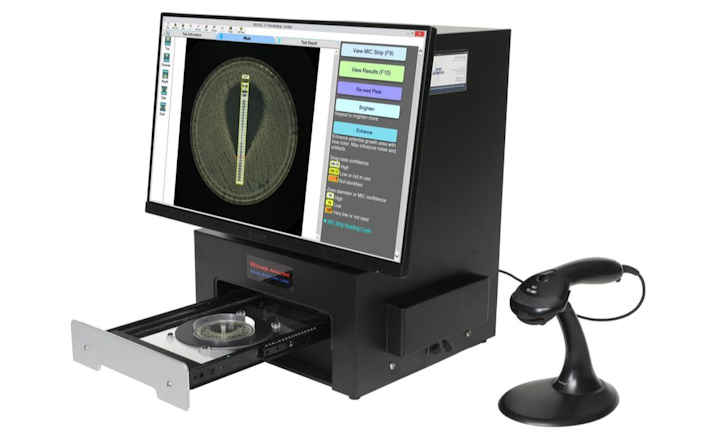
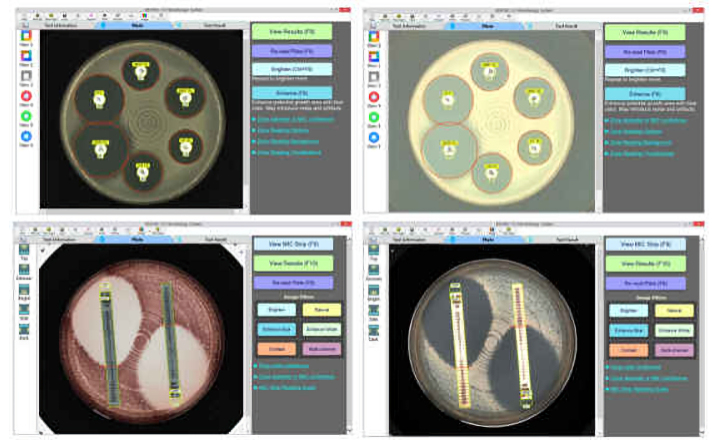
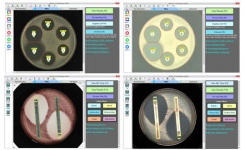
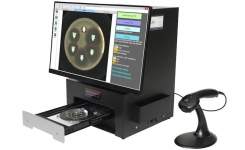
TRINITY V3 (Giles Scientific USA) utilizes digital imaging to automate zone reading and calculations for antibiotic potency assays following USP 81, EP, and JP methods.
This industrial microbiology system is used primarily in pharmaceutical QA/QC microbiology laboratories. TRINITY V3 is designed and manufactured by Giles Scientific in California, USA.
TRINITY V3 features and benefits include:
- 21 CFR Part 11 compliant
- Follows USP, EP, JP methods
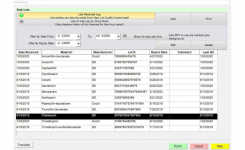
- IQ, OQ, PQ documents included
- No system maintenance
- Instant zone reading & assay calculations
- Customize plate layout and report
- Save high resolution plate images