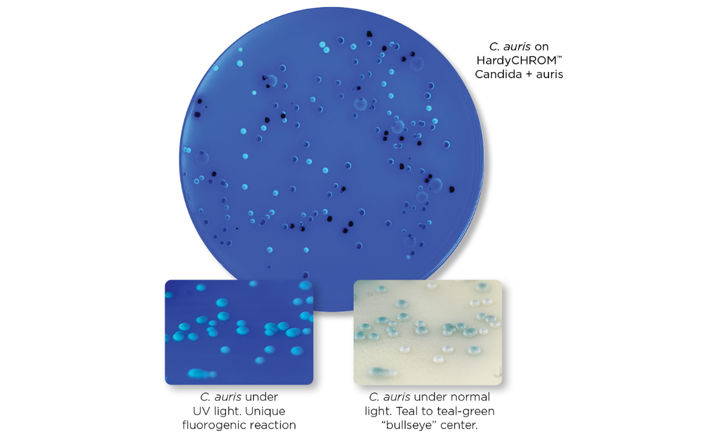
Hardy Diagnostics HardyCHROM™ Candida + auris is recommended for the selective isolation and differential identification of Candida species. The medium allows for the differentiation of C. albicans, C. tropicalis, C. krusei, and C. auris based on colony morphology, color, and positive UV fluorescence.
Colonies of Candida glabrata can be subbed directly to Rapid Trehalose Fermentation Broth (Cat. no. Z205) or GlabrataQuick (Cat. no. Z298) for confirmation
Key Points:
- Easy colony detection
- Candida auris produces white colonies with teal to teal-green “bullseye” centers that are positive for UV fluorescence
- Colonies stand out
- Bright, vivid color development
- Highly selective
- Useful as an aid for differentiating other clinically relevant Candida species (C. albicans, C. tropicalis, C. krusei).
- Fast development
- Most strains will exhibit colors within 48 hours