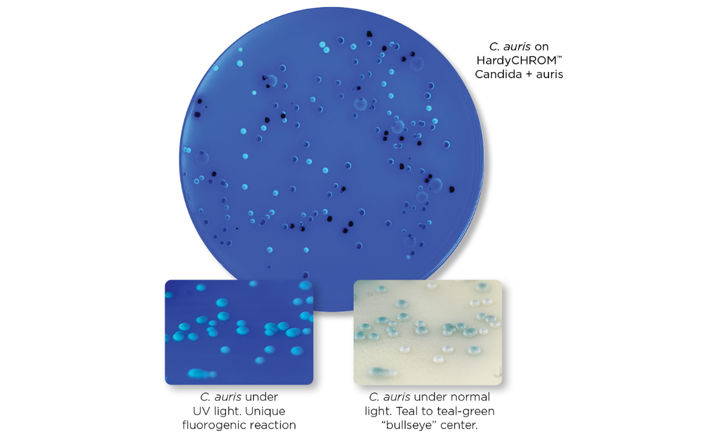
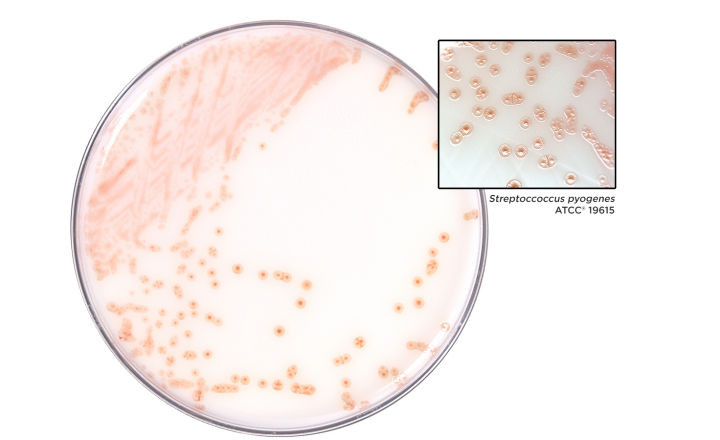
Strep B Carrot Broth™ One-Step is a selective and differential medium that is intended for the detection of Group B Streptococcus (GBS) from anovaginal specimens collected from pregnant women. The medium is used as an aid in the qualitative determination of GBS colonization in pregnant women. The color change reaction from white to orange is representative of a positive result for presence of hemolytic GBS.
Our one-step test is an improvement over conventional methods by increasing sensitivity, decreasing turn-around time, and lowering overall cost. All components are now included in the color change test, so the use of additional tiles is no longer necessary. Positives require no follow-up testing!
Key Points:
- One Step!
Easy one-step process! Place sample directly in the tube. Adding tiles is no longer necessary. - Simple read-out
Development of any orange to red color whatsoever indicates a positive result. - Accurate results
For positives, there is no need for further sub-culturing or testing. - Low cost
Lower overall cost when compared to LIM broth. - Easy to use
Can be used with liquid and gel-based transport systems. - Multiple formats
Carrot Broth media is available in multiple formats that work on many automated inoculating machines, such as the WASP®, BD Innova® and Kiestra™.
Click here for further information