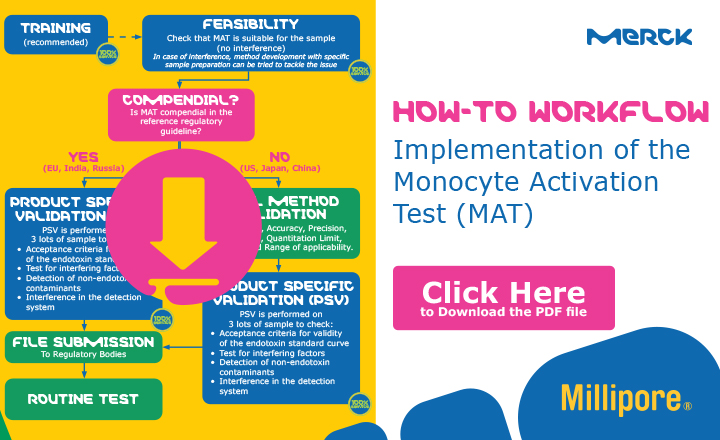
The European Pharmacopoeia Commission recently decided to revise the parts of 59 Ph. Eur. that refer to the rabbit pyrogen test, putting the Monocyte Activation Test (MAT) on track as its replacement in the EU. This in vitro test, which uses human monocytic cells to mimic the human reaction to pyrogens, was introduced into the EP back in 2010 (test specification 2.6.30) and is also compendial in Russia and India. In none of these countries is there a need to perform full method validation.
However, implementing the MAT regularly requires product-specific validation, and training is highly recommended. Having offered our own PyroMAT® system for several years now, we have gained the expertise and experience to guide pharmaceutical companies through the implementation process, for which we suggest the following workflow
Among the services and trainings, we offer for MAT implementation are method development and product-specific validation services, feasibility studies, e-learning, training, consultancy, and software-based data analysis for routine testing, with a choice of face-to-face or remote services for many of the modules.
Contact the Supplier using the green "Request Information" button below.