US food safety experts Richter International, a Matrix Sciences company, performed a study to evaluate the performance of the Solus ONE single enrichment immunoassays in the detection of Salmonella and Listeria from real world environmental samples. Solus One assays provide a next day negative or presumptive positive result thus enabling prompt corrective actions where contamination is found.
Solus One Salmonella and Solus One Listeria were compared with Solus Salmonella and Solus Listeria (dual enrichment ELISA assays) respectively. For the evaluation Dual Polyurethane sponges with Hi-Cap™ neutralizing buffer were used to collect environmental samples in a food manufacturing facility. The samples were collected from forty locations across Zone 2, 3, and 4 sites and then inoculated with a low level of the relevant pathogen. The sponges contained a mixture of organic material, competitor flora, and dry sanitizer (if present at swab site). The sponges were held overnight at refrigerated temperature to simulate shipping to an outside laboratory and then assayed using the respective protocols. At the end of the incubation time all the sponges were culturally confirmed.
Results:
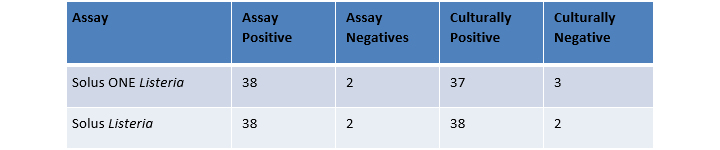
Visual examination of the negative samples showed a large amount of growth in the sponges. The cultural results yielded a wide variety of atypical colonies suggesting that these samples had a significant bioburden that might have suppressed the growth of Listeria. The unconfirmed assay result from Solus One Listeria was from a sample with a very low inoculation level (~1 cfu/sponge).
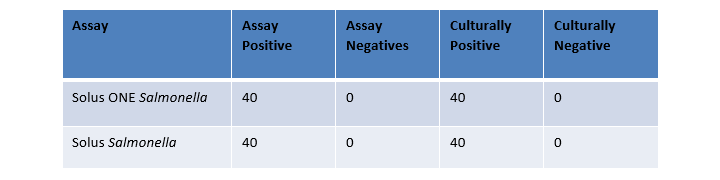
The study demonstrated that both Solus One assays are a robust alternative to a traditional dual enrichment assay equivalent. Solus One enables a faster turnaround time for environmental results thus allowing manufacturing facilities to take corrective actions sooner.