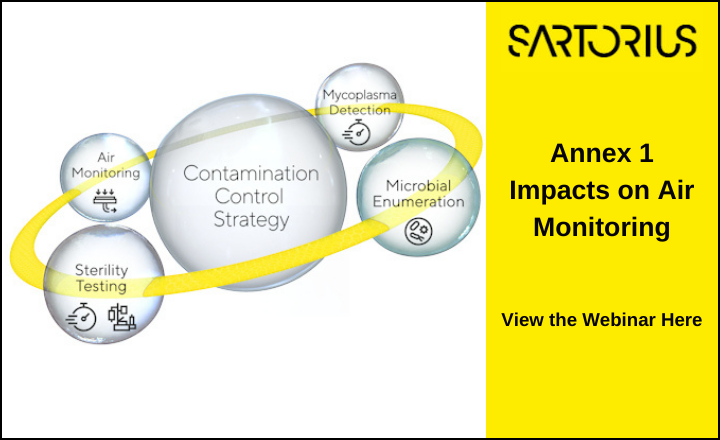
For professionals in pharmaceutical and medical device manufacturing, contamination is a pervasive concern that can disrupt production and compromise patient safety. The updated EU GMP Annex 1, with its focus on Quality Risk Management (QRM) and Contamination Control Strategy (CCS), has made it clear that a well-structured CCS is not just beneficial but essential.
Microbial contamination, particulate contamination, and chemical contamination are the primary adversaries in a sterile manufacturing environment and may render a product unsafe.
Developing a CCS requires a proactive approach. It begins with a risk assessment to identify potential contamination sources. Preventive measures are then established, including sanitation practices, air filtration systems, and personnel training. Continuous monitoring is essential to verify the effectiveness of these measures, and if contamination occurs, immediate corrective actions are necessary.
Regulatory agencies such as the FDA and EMA have set forth rigorous CCS requirements. The FDA demands a written CCS that details the company's strategies for preventing, detecting, and addressing contamination. The EMA also mandates a robust CCS, emphasizing a risk-based approach to prioritize high-risk areas
Sartorius offers reliable solutions that align with an effective CCS. The MD8 Airscan and Gelatine filters facilitate continuous air monitoring, while the Sterisart® family enable reliable sterility testing. The Microsart® @filter and @media system aid in bioburden testing by allowing touch-free membrane transfer. Additionally, Sartorius’ Microsart® qPCR kits provide rapid microbial testing, delivering results in just three hours, thus minimizing production line impacts.