Enumerate and presumptively identify Listeria species and L. monocytogenes from food and environmental samples with the Thermo Scientific™ Oxoid™ Brilliance™ Listeria Agar (ISO) prepared bottles as part of the Thermo Scientific™ Listeria Precis™ Enumeration method.
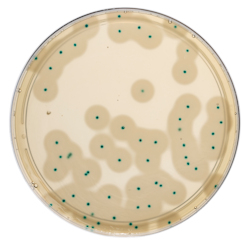
Listeria monocytogenes are further differentiated by their ability to highly produce lecithinase producing an opaque, white halo around the colony.
Pour plating is particularly of interest to enumerate low contamination levels using a single Brilliance Listeria Agar (ISO) plate compared to multiple plates needed when following the ISO 11290-2 method for enumeration. Pour plating has shown equivalence to the ISO 11290-2 reference method with an ISO 16140-2:2016 validation study by AFNOR.
With the Listeria Precis Enumeration Method, results are available in as little as 24 hours, with confirmation in around 15 minutes when using the Thermo Scientific™ PrecisCheck™ Listeria species or L. monocytogenes lateral flow strips.*
Already available as dehydrated culture medium and ready-to-use supplement agar plates, the new ready-to-melt agar bottles will be available in January 2024 in packs of 10 x 200 mL. No weighing, mixing or autoclaving is needed. Simply melt, supplement, and pour the cooled, molten agar into Petri dishes with the diluted sample and incubate. Plates are inspected at 24 - 48 hours and any presumptive Listeria species and L. monocytogenes colonies counted for the enumeration calculation.
Listeria Precis Methods are the next generation in culture media-based testing with a simplified workflow compared to other culture media methods. Superior performing culture media means a simpler workflow, easier-to-read plates, and fewer presumptive-positive colonies to confirm.
To find out more about the Listeria Precis Detection and Enumeration Methods and other products for the workflows visit thermofisher.com/listeria-precis.
*Thermo Scientific™ PrecisCheck™ Listeria species or L. monocytogenes lateral flow strips are not available in all countries. Alternative confirmation methods are available. Please contact your local supplier to discuss.