We are delighted to announce that on December 4th, 2018 the AFNOR technical board granted Solus One Salmonella, NF validation certification. The method was compared to the reference method ISO 6579-1 (2017) following the validation protocol ISO 16140 (2016) for the detection of Salmonella in the following food categories: ready to eat, ready to reheat (excluding smoked products), heat processed milk and dairy products, and egg products.
This complements AOAC Performance TestedSM Method (PTM) certification, which was achieved in October 2018. The study demonstrated the kit to be equivalent to FDA BAM Chapter 5 for the detection of Salmonella from raw salmon, cheddar cheese, romaine lettuce, non-fat dry milk powder and selected environmental surfaces. And equivalent to the USDA laboratory manual 4.09 Isolation and Identification of Salmonella for detection from raw beef trim and pasteurized eggs.
To further support these claims we are also validating additional food matrices. In December our R&D team completed a study for the detection of Salmonella in raw meats. This study evaluated thirty-four diverse samples as representative of products within the raw meat food category. The method was compared to the reference method ISO 6579-1 (2017) and meets the acceptability criteria for this food category.
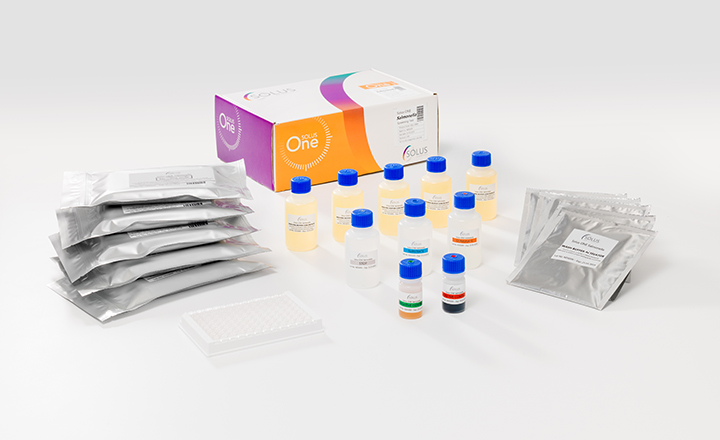
Solus One Salmonella is a highly efficient assay for the next day detection of Salmonella in selected food and environmental samples.
Benefits:- Next day results.
- Positive confirmation possible in additional 24 hours.
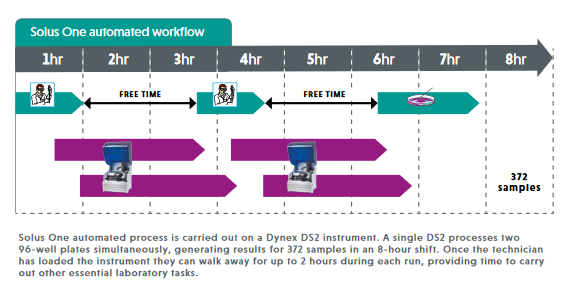
- Highly efficient automated assay, significantly reducing technician hands on time.
- High sample throughput can be achieved with a single instrument.
- Ability to cope with fluctuating sample volumes and capacity to grow.
- Excellent sample traceability when using the on-board bar-code facility.
- Small instrument footprint maximises bench space, compact product packaging reduces cold storage space requirement and packaging waste when compared to other methods.
- AOAC PTM certification plus independent validation demonstrating excellent results when compared Solus dual enrichment ELISAs.
Solus One – More than fast
Visit www.solusscientific.com/solus-one