The food industry has adopted a preventative, proactive approach to correcting potential safety issues. As part of that approach, food manufacturers should use additional monitoring methods to determine the general hygiene of food processing areas. This includes microbiological testing which must be done to verify the effectiveness of any EMP. However, it can often be difficult to test large areas or batches of product. In cases where a full-scale test isn’t possible, or for more frequent testing, it can be beneficial to test for organisms whose presence tends to coincide with pathogens, contamination, or spoilage. These organisms are called indicator organisms, and they can act as early warning signs.
Historically, three groups of coliforms have been used as indicators but in different applications. Detection of total coliforms is used as an indicator of water quality or as a general indicator of sanitary conditions in food-processing environments. Fecal coliforms remain the standard indicator of choice for shellfish and harvest waters, and E. coli is used to indicate fecal contamination or unsanitary processing.
While strict, defined operations can help prevent contamination, they don’t ensure that the final product is free of all possible pathogens. Therefore, it is critical to include indicator organism testing as part of a facility’s EMP. For each indicator, simple pass/fail criteria can be established from a facility’s historical data. The EMP and the target/baseline are unique for each plant, each product type, and the baseline varies across zones. Many facilities rely on traditional culture methods for contamination detection. One of the most common indicator tests is Total Plate Count (TPC).
TPCs are time-consuming and require additional plating and biochemical testing to identify specific species or strains. After days of testing, if positive results for a pathogen are obtained, products made on that line must be held until further confirmative test results are available, and it will most likely initiate a recall situation or discarding of precious product. This increases the overall costs for the facility – discarded product, line stoppage, additional cleaning, and retesting.
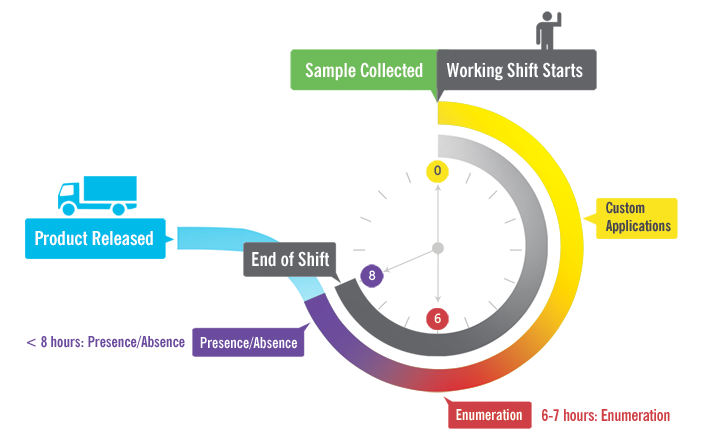
As an alternative to plating, facilities should consider more rapid testing to identify the presence of any indicator organisms. Tests must be simple, sensitive, accurate, and provide results as quickly as possible, preferably within a shift (6 - 8 hours). One solution meeting these criteria is MicroSnap™ rapid test devices. Tests are available for total viable counts (TVC), coliforms, E. coli, and Enterobacteriaceae.
MicroSnap is a novel, rapid test system capable of detecting bacteria using a modification of the adenosine triphosphate (ATP) bioluminescence reaction, which most processors are familiar with from routine ATP sanitation monitoring. These assays can detect bacteria at low levels in a variety of sample types, providing results in 6-8 hours when read using the easy-to-use, sensitive, accurate EnSURE™ Touch luminometer from Hygiena™. The MicroSnap light-generating signal can be quantified in the multi-functional EnSURE Touch system and stored in a cloud-based software application (SureTrend™) for further analysis, reporting and trending. The system has been AOAC-RIPTM validated for the enumeration of total bacteria (TVC), coliforms, and E. coli.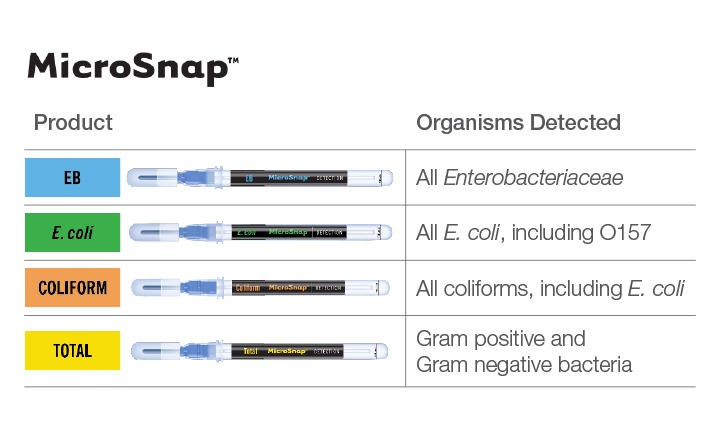 Implementing this simple, rapid, low-cost test system can provide you with results in the same working day or shift – ideal for monitoring the quality and safety of food as well as post-cleaning surface hygiene. This platform uses the same foundation of traditional pour plates or sample-ready media film with an enrichment step that allows the microorganisms to grow.
Implementing this simple, rapid, low-cost test system can provide you with results in the same working day or shift – ideal for monitoring the quality and safety of food as well as post-cleaning surface hygiene. This platform uses the same foundation of traditional pour plates or sample-ready media film with an enrichment step that allows the microorganisms to grow.
Instead of counting colonies, MicroSnap uses a unique formulation and rearrangement of the ATP bioluminescence biochemistry coupled to specific substrates to detect specific bacteria at low levels in just a few hours. These tests detect between 1–10,000 CFU, whereas sample-ready media film typically detects between 10–300 CFU. The test is flexible for environmental sampling with a built-in sample collection swab for assessing surface contamination and environmental hygiene. The unique self-contained packaging format simplifies the technology, so the test is simple and easy to use in any facility by any user. In addition, the use of this platform reduces costs, especially for facilities using outside laboratories for these quality tests.
Contact us for more information.