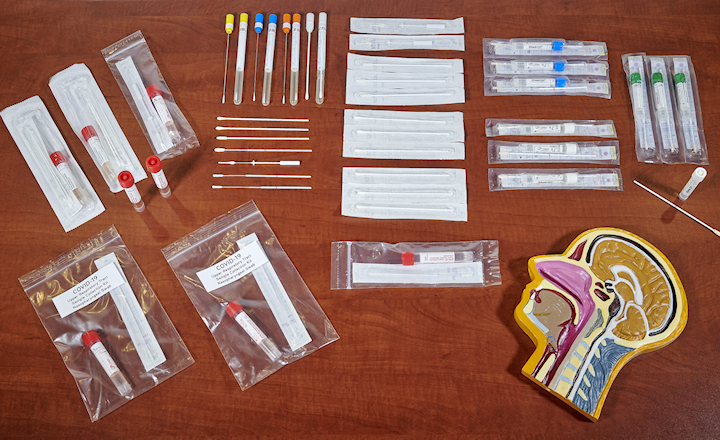
In this video, Norman Sharples, COPAN Diagnostics’ CEO, discusses the different sites and swab materials available for sample collection for COVID-19 based on the FDA Guidelines as of April 1, 2020. FDA believes that a nasopharyngeal specimen is a preferred choice for swab-based SARS-CoV-2 testing. If a nasopharyngeal specimen is not available, then any of the following are acceptable: Oropharyngeal specimen collected by a healthcare professional (HCP); Mid-turbinate specimen by onsite self-collection or HCP (using a flocked tapered swab); or Anterior nares specimen by onsite self-collection or HCP (using a round foam swab or polyester). The situation regarding the types of sample collection kits acceptable for COVID-19 testing remains fluid.
Consult the current guidance for information on sample collection systems at the FDA Medical Devices Emergency Situations - FAQs on Diagnostic Testing for SARS-CoV-2: