ProGnosis Biotech has picked up Gold and Bronze at the recent 2021 Healthcare Business Awards. The awards are given out each year in recognition of excellence in healthcare and innovation and investment in Greece. Prognosis scooped the top prize in the 'Medical Equipment Company' category for its high growth rate for a new company in the field of clinical diagnostics. It also picked up bronze in the 'Technology and Innovation' category for manufacturing the only homegrown rapid test for COVID-19.
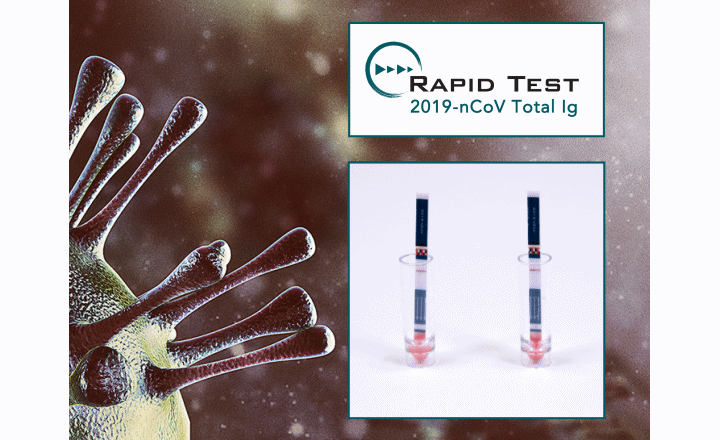
Part of Prognosis Biotech's COVID-19 test portfolio is a Rapid test 2019-nCoV Total (V1210/V1230) for the detection of IgA, IgM, and IgG antibodies against SARS-CoV-2 in human serum, plasma, or whole blood specimens.
The company participated in a clinical performance study, which was conducted on June 12, 2020, at the School of Medicine, of the Aristotle University of Thessaloniki, which serves as one of the official reference laboratories for SARS-CoV-2 in Greece. For the evaluation, 80 positive and 114 negative, samples were examined (194 in total) and
The Rapid test 2019-nCoV Total Ig demonstrated 98,75% sensitivity and 100% specificity for the detection of total antibodies against SARS-CoV-2. What is more, for samples that were collected after the first 7 days from the onset of the symptoms, both sensitivity and specificity reached 100%.
Advantages:
- Lateral flow in dipstick format available in 10 or 30 test sticks
- Low procedure time: 10 min
- Easy-to-use method, no need for equipment or special facilities
- Visual interpretation of the results
- Shelf Life: 12 months | Storage 4-30°C
Request information using the button provided below or visit Prognosis Biotech's website to learn more.