Global Risk-Based Allergen Thresholds
Food allergens remain a major driver of product recalls – undeclared allergenic ingredients account for roughly 30% of food recalls, with major implications for public health and business risk. Unlike pesticide residues or veterinary drugs which are regulated by strict established Maximum Residue Limits (MRLs), there are no official regulatory thresholds for allergen residues. Instead, risk-based frameworks guide allergen management.
International bodies like WHO/FAO have introduced reference doses (RfDs) for priority allergens (for example, about 2 mg of protein for milk or egg), below which the vast majority of allergic consumers are protected. Likewise, the VITAL 3.0 program uses ED05 thresholds (doses expected to affect only 5% of allergic individuals) to set action levels for labeling. These benchmarks inform when precautionary allergen labels are warranted. WHO/FAO further recommend that analytical methods achieve a Limit of Quantification (LoQ) at least three times lower than the reference dose, ensuring even trace-level allergens can be detected reliably.
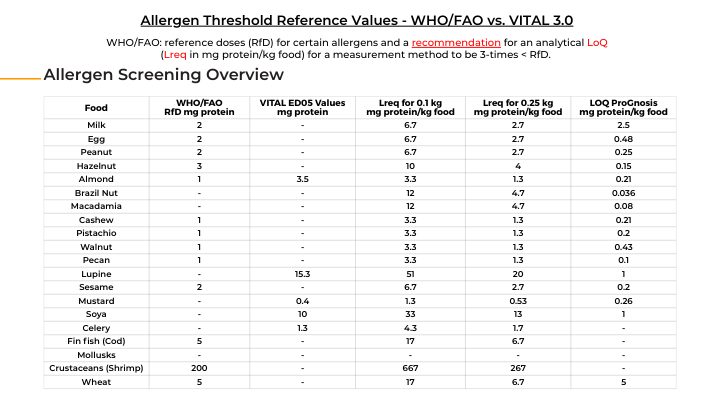
Download the Allergen Threshold Reference Values here.
Allergen Free & Allergen Shield Series
To comply with these stringent standards, ProGnosis Biotech offers two complementary allergen detection solutions for quantitative testing:
Allergen Free Series (Lateral Flow):
- Detects 17 common allergens in a cassette format: soy, gluten, milk, nuts and others.
- 1 extraction buffer and unified protocol for all allergens simplify workflow and training.
- Suitable for testing food, CIP solutions, and working surfaces in just 5 minutes.
- Portable reader pairs with an Android app, providing immediate, shareable quantitative results.
- Incorporates a hook line on test strips to prevent false negatives at high allergen concentrations.
Allergen Shield Series (ELISA):
- Offers quantitative analysis of 22 allergens such as gluten, milk, soy, crustaceans, fish, nuts and others.
- Uses highly specific monoclonal antibodies to minimize cross-reactivity and ensure accuracy.
- Standardized 30-minute testing protocol across kits ensures consistency and ease of use.
- Includes surface swabbing kits to verify cleaning effectiveness and hygiene.
Together, these platforms enable both rapid in-plant screening and sensitive lab confirmation of allergen levels. They can be integrated into allergen monitoring plans (e.g., HACCP or ISO 22000 programs), helping manufacturers verify compliance with global safety standards and risk-based labeling guidelines.
Hygiene Verification & Data Connectivity
Both allergen testing systems can be paired with the ATP-FLOW surface ATP test to verify equipment cleanliness before production. This pre-operational hygiene check ensures that any allergenic residues are removed prior to processing. In addition, the portable 3PR Mini reader syncs via Bluetooth with a mobile app to log results (with time stamps and GPS location), enabling real-time traceability and remote monitoring of cleaning and testing data.
The ProGnosis Biotech Advantage
Certified under ISO 9001, ISO 13485, and ISO 37001, ProGnosis Biotech combines scientific reliability, sustainable testing protocols, and digital connectivity—helping manufacturers and labs stay compliant, efficient, and trusted in a demanding global food safety landscape.
























