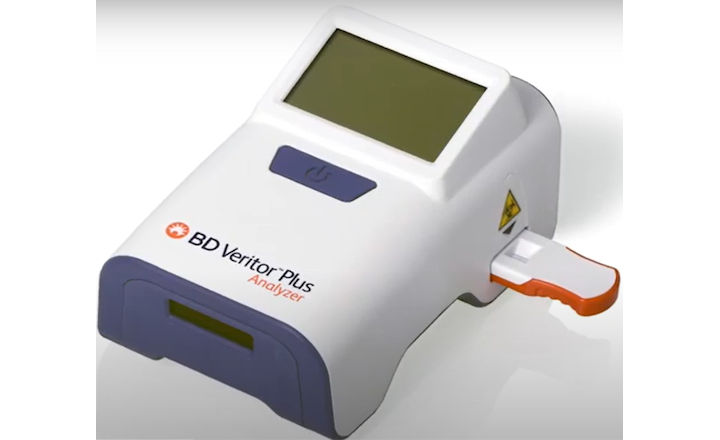
BD (Becton, Dickinson and Company) has announced CE-marking for its rapid, point-of-care, SARS-CoV-2 antigen test for use on the BD Veritor™ Plus System.
The new test delivers results in 15 minutes on an easy-to-use, portable instrument, which is a critical improvement in turnaround time for COVID-19 diagnostics because it provides real-time results and enables decision-making while the patient is still onsite.
The company expects commercial availability of this new assay at the end of October for countries in Europe that recognize the CE mark.
The test, which has been available in the United States since July through an Emergency Use Authorization by the U.S. Food and Drug Administration (FDA), uses the BD Veritor™ Plus System, which is already in use across Europe to test for conditions such as Group A Strep, influenza A+B and Respiratory Syncytial Virus (RSV).
The BD Veritor™ Plus System, which is slightly larger than a mobile phone, offers an easy-to-use workflow that makes it an ideal solution for point-of-care settings. It also offers customers traceability and reporting capabilities through the optional BD Synapsys™ informatics solution.
The European Centre for Disease Prevention and Control (ECDC) recently released guidance that all patients with acute respiratory symptoms should be tested for both SARS-CoV-2 and influenza A+B in parallel during flu season.
The BD Veritor™ Plus System can test for both infections on the same platform. BD is leveraging its global manufacturing network and scale to produce 8 million SARS-CoV-2 antigen tests per month by October and expects to produce 12 million tests per month by March 2021.
Note: This content has been edited by a rapidmicrobiology staff writer for style and content.