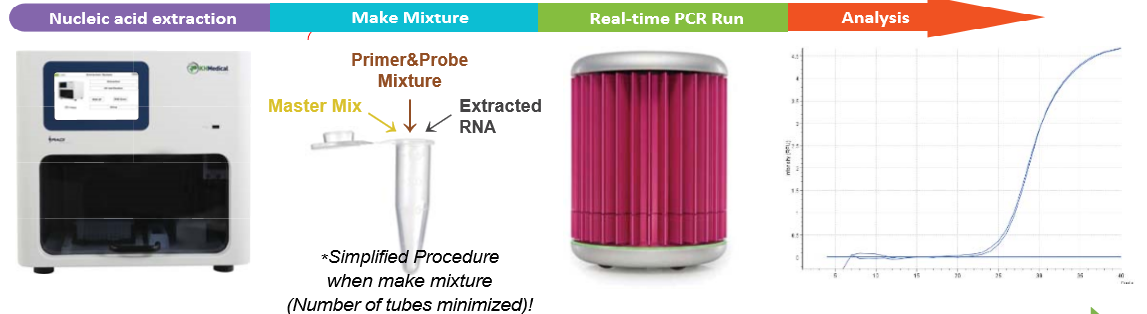
KH Medical (Rep. of Korea) has announced that its RADI COVID-19 Real-Time PCR Detection Kit has received CE-IVD marking. The kit is used for the qualitative detection of SARS-CoV-2 in human nasal swab or sputum by using RADI Prep Plus (fully automated extractor) and RADI Real-Time PCR system and runs on all major thermocyclers, i.e. ABI 7500 or Bio-Rad CFX 96 Platform.
The assay has two targets as part of its assay for SARS-CoV-2; the S-gene (spike glycoprotein) and the RdRP gene (RNA-dependent polymerase). It has a limit of detection (LOD) of 0.66 copies/µL and can provide a sample-to-result turnaround in 80 mins.
Please note : Any products described on this page are
for Research Use Only and not intended for clinical diagnostic procedures unless otherwise stated.





















