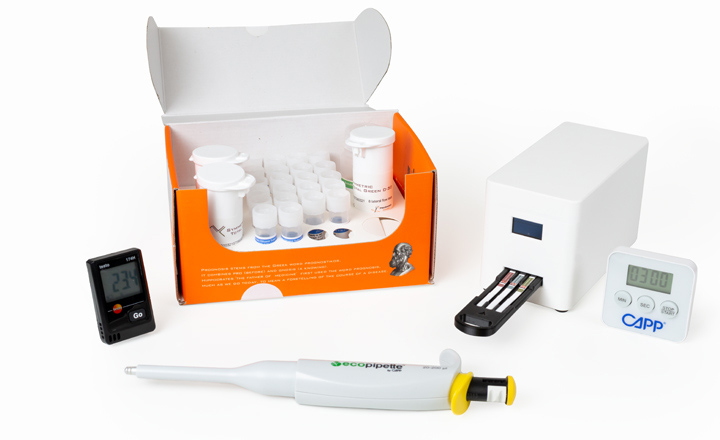
One of the most common analyses performed on fish, especially on pelagic species, is the detection and quantification of histamine. Although it is considered an allergen, it is rarely mentioned alongside other regulated foodborne allergens, such as gluten, soya, etc.
Presence of histamine is related to fish degradation due to inadequate storage after fishing; therefore, it is mainly related to wild-caught fish. As storage conditions improve, although boat crews still must go out in the ocean and temporarily store fish in their fridges, histamine levels have been significantly reduced. Last December, the FDA further reduced the MRL of histamine in the US from 50 to 35 ppm, signifying that the industry has managed to contain what seemed to be a major issue a decade ago.
Regardless that histamine levels are constantly being reduced, testing continues to be rigorously implemented worldwide, where fish is caught and canned. Therefore, testing needs to take place at two points: entry of raw materials and on the finished product.
The most common method in the industry for the past 15 years has been ELISA, since HPLC can test just a limited number of samples per day. Additionally, the cost per sample is quite high in comparison to immunoassays. Therefore, quality control on histamine has been traditionally related to ELISA, deterring most manufacturers from investing in a Quantitative Lateral-flow test.
The only way to displace ELISA from a lab, is to offer the capability of accurately quantifying histamine in a variety of pelagic fish, faster and simpler. Users that are accustomed to ELISA find it very pleasant to move to a much simpler and significantly faster method, where actions don’t have to be delicately performed, but are usually puzzled in relation to the accuracy and reproducibility of the lateral-flow test.
When it comes to Histamine, it’s not all about a “pass or fail” result, but the exact quantification of a substance regulated at low limits due to its allergenic features. Moreover, when considering that canned fish is a value-added product, compared to meat, canning industries become quite stringent with the raw materials they purchase.
ProGnosis Biotech would like to present the first Quantitative Lateral-flow to be validated by AOAC, without any acylation step, with a simple Distilled Water extraction and just 3 minutes to result.
Symmetric Histamine is a fully quantitative lateral-flow test, with a very simple and fast protocol, using distilled water for extraction, suitable for testing at the lab, or at the port or factory, at any reception point. In terms of accuracy, Symmetric Histamine is the only Quantitative Lateral-flow which participates twice per year in Fapas Proficiency Tests on canned tuna, sardines and anchovies, in oil, water or sauce.
Contact ProGnosis Biotech for all documentation related to the product or click on the Request Information button below.