Selective agar-based media are commonly used to classify microorganisms in microbiology and medical diagnostics, enabling growth of the microbes of interest while inhibiting other organisms. Charcoal agar, for example, is used for the cultivation and isolation of Bordetella pertussis – responsible for the severe respiratory infection whooping cough – and Haemophilus influenzae, a known cause of meningitis, predominantly in young children. Other media, such as blood agar, are designed to recognize bacteria based on the color of the colony. Blood agar is commonly used to classify streptococcal species, which are responsible for many cases of bacterial pneumonia, meningitis, endocarditis and other serious infections. The quality of the blood agar plates is of the utmost importance for reliable determination of the hemolysis type.
In this exclusive interview with rapidmicrobiology, Dr Ursula Leuthold, global product manager for media preparation devices at INTEGRA Biosciences, gives an insight into how the company’s MEDIACLAVE media sterilizer and MEDIAJET Petri dish filler, together with a DOSE IT peristaltic pump, can help laboratories to meet the challenge of preparing high quality, selective agar.
What are the challenges of preparing selective agar?
Some media, such as charcoal agar, are very viscous and usually quite time consuming to prepare, as pre-swelling is necessary. For other media, like blood agar, maintaining the correct dispensing temperature is essential to minimize thermal stress to the erythrocytes; if it is too hot, the cells will lyse, identified by the agar changing from light red to brown in color. Equally, if the dispensing temperature is too cold, the mixing process is not as efficient, resulting in uneven distribution of the blood on the plates, evidenced by streaking. INTEGRA’s MEDIACLAVE offers the perfect solution to overcome these challenges. It enables continuous, bi-directional stirring that improves the mixing performance of viscous media, as well as minimizing foaming and swelling times, and a low dispensing temperature can be set and maintained.
How can additives be mixed into the agar?
Additives are often temperature sensitive, and cannot be sterilized together with the agar. A very good option for antibiotics or dyes is to add them to the medium after sterilization. However, this is not suitable for blood cells, which must be mixed into the agar during dispensing, typically at a concentration of between 5 and 10 %. It is essential that the agar-to-blood volume ratio is always the same, and that the agar is maintained at a temperature between 42 and 47 °C to avoid lysis of the erythrocytes. In addition, refrigerated blood must be tempered to room temperature before mixing to preventing partial gelling of the agar.
MEDIACLAVE allows the injection of supplements, for example, antibiotics, via a self-resealing septum while maintaining the agar at exactly the required dispensing temperature. In combination with the MEDIAJET Petri dish filler and DOSE IT peristaltic pump, any additive can be continuously mixed into the agar at the required concentration. Blood can be added to the agar immediately before pouring the plate, significantly reducing blood cell lysis, taking advantage of the precise synchronization between the MEDIAJET and the DOSE IT pump to ensure easy, correct setting of flow rates, guaranteeing the essential constant blood volume dispensing.
Why should you automate media preparation?
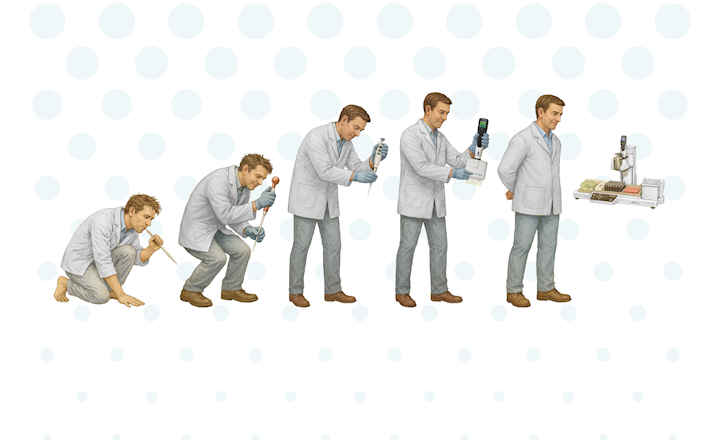
Automation offers a number of advantages over manual processes, not least the elimination of manual errors, standardization of protocols, and improved reproducibility and quality control. A sterile environment is of the utmost importance when dispensing media into Petri dishes and tubes, and this can easily be jeopardized during manual processes; with automation, the potential to introduce contamination is drastically reduced. Manual processes are also tedious and time consuming, and walk-away automation frees up staff to undertake other, more interesting, tasks.
What are the key questions to ask a vendor?
Two of the most important considerations are an intuitive user interface and a robust design, since this will ensure downtime is minimized and productivity maximized. It is important to consider the after-sales services too; can the manufacturer offer good customer support, including installation and servicing, timely repairs, and operator training?
Key considerations when choosing an automated media dispenser are:
- Reliability - how frequently will it need servicing and how quickly can a service engineer attend?
- Automation - does it offer true walk-away automation?
- Bar coding - can batch details be printed on the dish?
- Footprint - will it actually fit in the lab space?
- Contamination - what measures are in place to prevent cross-contamination occurring?
It is also important to think about the size and shape of plates that the system must handle, the dispensing volume range, whether it is straightforward to switch from dispensing into plates to filling test tubes, and how easy it is to thoroughly clean the filling chamber.
How does the INTEGRA range meet the needs of a laboratory?
INTEGRA’s MEDIACLAVE, DOSE IT and MEDIAJET, complemented by the optional TUBEFILLER add-on, enable straightforward preparation and dispensing of culture media into 90, 60 or 35 mm diameter Petri dishes, as well as 13, 16, 20, 25 and 30 mm diameter test tubes. A worldwide network of highly trained service partners guarantees access to valuable expertise and high quality after-sales support.
The MEDIACLAVE media sterilizer guarantees the highest safety standards, with software that monitors temperatures and pressures throughout the run, automatically shutting down and cooling if these surpass a certain threshold. An overpressure safety valve and burst disc release excess pressure if all the other safety systems fail. The system also has an automatic indicator of service intervals, and tightness can be easily checked for efficient operational safety. These sophisticated safety features set the MEDIACLAVE apart from other media sterilizers.
Process documentation (i.e. run files) can be automatically stored online through the USB port or printed using the optional feature of a built-in printer. Log files can be stored electronically without extra software via an ethernet connection and will comply to FDA (21 CFR Part 11) and EU (GMP Annex 11) protocols using a digital signature, unlike competitor systems.
The MEDIACLAVE is one of the fastest media sterilizers on the market because of its very efficient cooling system, taking less than one hour for the normal 20 minutes at 121 °C run. The water jacket between the media container and the pressure vessel ensures uniform heating, perfect sterilization and reduces chances of the contamination.
MEDIAJET
 The MEDIAJET media filler offers true walk-away automation, using Feed-In/Stack-Out technology to deliver reliable and completely user-independent operation. Petri dishes of varying dimensions are easily accommodated, held securely by a 'gripper' system to ensure a more stable transfer, and avoiding the potential for plates to fall that is inherent in tray-guided systems. MEDIAJET is equipped with the fastest rotor-carousel available, capable of filling 900 plates an hour in the standard mode and 1,100 using the turbo set-up. A UV lamp integrated into the loading area means that plates are opened and closed in a sterile environment, reducing the likelihood of contamination. These key features differentiate INTEGRA’s MEDIAJET from alternative products on the market.
The MEDIAJET media filler offers true walk-away automation, using Feed-In/Stack-Out technology to deliver reliable and completely user-independent operation. Petri dishes of varying dimensions are easily accommodated, held securely by a 'gripper' system to ensure a more stable transfer, and avoiding the potential for plates to fall that is inherent in tray-guided systems. MEDIAJET is equipped with the fastest rotor-carousel available, capable of filling 900 plates an hour in the standard mode and 1,100 using the turbo set-up. A UV lamp integrated into the loading area means that plates are opened and closed in a sterile environment, reducing the likelihood of contamination. These key features differentiate INTEGRA’s MEDIAJET from alternative products on the market.
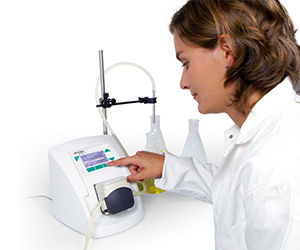
DOSE IT has been specially designed for laboratories looking for an easy-to-use, compact and portable peristaltic pump. With its lightweight and compact design, it fits anywhere in the lab, can be easily moved and is easy to operate via the intuitive user interface. DOSE IT is especially helpful for dispensing culture media, as the transfer liquid remains enclosed inside the tubing, minimizing the risk of contaminating either the product or the pump itself.
TUBEFILLER

Microbiology labs not only grow bacteria on agar plates, but also culture microorganisms in suspension in tubes, typically using peristaltic pumps, such as INTEGRA’s DOSE IT, to dispense the broth media. The optional TUBEFILLER is the perfect extension to the MEDIAJET, adding extra functionality and increasing the system’s flexibility. This unique solution allows quick and easy conversion of the automated Petri dish pourer into a test tube filler, making the MEDIAJET the most versatile media dispensing system in the market. TUBEFILLER can continuously process racks accommodating test tubes of various diameters and length, and is suitable for a wide range of applications, including the production of agar slants, broth cultures or sodium chloride dilutions.
Is it possible to see the MEDIACLAVE, MEDIAJET, DOSE IT and TUBEFILLER live?
Yes, of course! Seeing the systems in action helps you to understand the benefits of automated media dispensing into plates and tubes. Visit the INTEGRA website to arrange a live demonstration or to request more information about automated media dispensing with INTEGRA’s MEDIACLAVE, DOSE IT, MEDIAJET and TUBEFILLER systems!
About Dr Ursula Leuthold

Dr Ursula Leuthold is currently global product manager for media preparation instruments at INTEGRA Biosciences. Her background includes an undergraduate degree in biology and a PhD in natural sciences.
Over the course of her career, Ursula has researched the co-stimulatory effects of connective tissue molecules on T cell activation, and has gained extensive experience in account and business management, technical writing and online marketing.