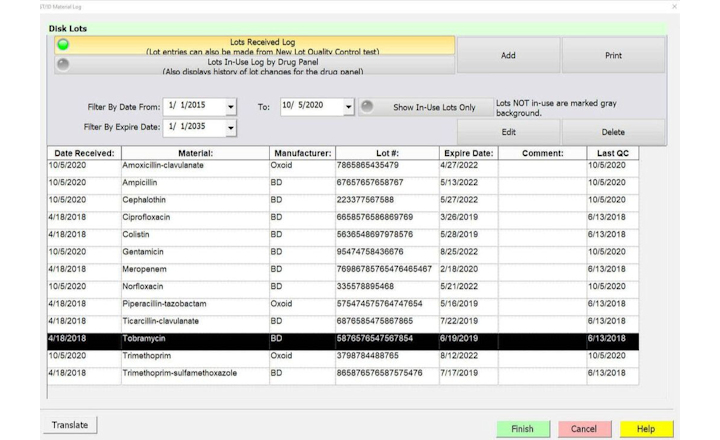
The T2Resistance Panel ™ developed by T2 Biosystems that can detect 13 of the most serious resistance genes in whole blood specimens has been granted CE-IVD marking and is now available in Europe. It is the first in vitro diagnostic test which detects these antibiotic resistance genetic markers from both gram-positive and gram-negative pathogens and gives results in under 5 hours, without waiting for a blood culture.
The Centre for Disease Control and Prevention (CDC) have recently released their 'Antibiotic Resistance Threats in The United States 2019' report which lists 18 organisms carrying these 13 mobile elements providing resistance to a clinician's arsenal of antibiotics.
The T2Resistance Panel received CARB-X funding for its development and was the funding body's first graduate from its portfolio. To receive CARB-X funding, T2 Biosystems had to demonstrate to an advisory panel consisting of global experts in the antimicrobial resistance (AMR) and diagnostic fields that the innovation had the potential to significantly accelerate and improve the detection and diagnosis of serious drug-resistant bacterial infections and to contribute to the global fight against drug-resistant bacteria.
In February of this year, the U.S FDA granted "Breakthrough Device" designation for the T2Resistance Panel, but as yet has been granted commercial clearance there and is only available for research.