According to WHO, the current dominating Delta variant is the "fittest and fastest of all SARS-CoV-2 strains" and is the most recent one classified by them as a Variant of Concern (VOC). Although COVID-19 vaccines are proving to be a tremendous asset in our fight against the virus, it would be logical to assume that if another VOC emerges, it would be an improvement on the Delta variant. So as restrictions lighten and funding tightens, public health labs will need to look for cost-saving solutions to maintain SARS-CoV-2 new and emerging variant surveillance and the high-throughput testing that has been the backbone of their defense.
Fluidigm, a US company known for introducing microfluidics to the life sciences, has launched a 'Targeted-Sequencing Assay Pool Set for the SARS-CoV-2 Genome' on its Juno™ platform; a bench-top system that uses Integrated Fluidic Circuitry (IFC) to automate molecular testing and library preparation for sequencing.
The panel will allow public health labs and vaccine developers to discover new mutations and follow transmission pathways, plus provide essential SARS-CoV-2 evolution data that can help to understand the emergence of new strains within a population or geographic area.
In this interview with Roberto Spada, Senior Manager of Strategic Marketing in EMEA at Fluidigm, he explains how much lab time can be saved by using the Juno™ to automate the ordinarily time-consuming and laborious step of library preparation when sequencing the SARS-CoV-2 genome, and goes on to explain how their nanolitre sample and assay method can save on reagents when testing for SARS-CoV-2 and many other infectious diseases.
Q. What is the difference between the Juno™ and other NGS library prep methods on the market?
One of the main differences is that the Juno™ is the only bench-top system that can completely automate the library preparation process for Next Generation Sequencing, whether it's for targeted NGS or for mRNAseq studies. What makes it unique is that it really simplifies the whole process since users need only to load in their assays and the samples onto our integrated fluidic circuits, or IFCs (which you can pipette just like classic 96/384 well plates), and the Juno then takes care of all the rest. This means that all the tedious, manual steps that are always involved in preparing libraries for NGS are completely automated by the Juno, leading to improved lab efficiency and more reproducible results. And all of this is done by a device that can just sit on any lab bench without taking up a whole room as some liquid handling robots do.
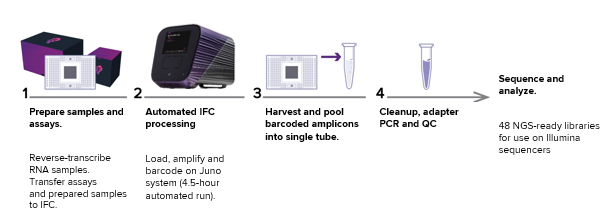
Fig 1. Typical workflow using the Juno™ and its IFC for preparing NGS-ready libraries for sequencing
Q. Where is the demand for such a high-throughput library prep platform?
I believe that the demand is out there, not only for a high-throughput library prep platform but for platforms that also provide a flexible workflow as well.
If we think about the amount of daily positive cases of SARS-CoV-2 worldwide, which are still in the thousands per day, there is a need to free up as much time as possible for any laboratory to be able to perform reflex-testing on SARS-CoV-2 samples to identify variants of concern. So while high-throughput capabilities are important, we must not forget that most testing laboratories don't only have to screen and analyze samples positive for SARS-CoV-2, but also patient samples for a variety of different diseases and conditions. Thus, the possibility of having a platform that could provide the flexibility of testing different samples, all by using the same workflow and instrumentation, could greatly increase efficiency and test turnaround times, which is crucial in today's world.
Q. Why would laboratories perform custom-targeted sequencing of the SARS-CoV-2 genome?
Once a patient has been tested positive for SARS-CoV-2, standard practice requires the sample to be tested again in order to identify which variant of SARS-CoV-2 the patient was infected with. By performing a custom targeted-sequencing panel for the SARS-CoV-2 genome, which provides uniform sequence coverage across the virus genome, the laboratory can be assured to be able to not only find known variants of concern but also be capable of uncovering novel variants that are known to arise due to SARS-CoV-2's high mutation rate.
Q. Can you give an example of a client who has used this technology for their microbiology/virology work?
A good example of this is actually coming from a customer of ours in France. They are part of the molecular biology service center in a university hospital and were already using our Juno system for targeted NGS screening of different tumor samples. However, when the SARS-CoV-2 pandemic arrived, they required an easy solution that would allow them to continue performing simple and fast NGS library preparation for the samples they received, but without having to completely change the workflow they were accustomed to. By simply adding our ready-to-use assay for targeted sequencing of the SARS-CoV-2 to their workflow, they were capable of simultaneously performing this automated library preparation for their oncology and SARS-CoV-2 studies, without changing instruments or chemistry and with no down-time.
Q. What are its throughput capabilities? Is it only for high-throughput labs?
Another unique feature of Juno is that it is a very versatile and scalable platform. It can be used for processing only a few samples per day up to hundreds of samples per day, depending on the needs of the laboratory. And all of this can be done without changing the chemistry or the workflow in the laboratory since all the user has to do is just load the pre-existing assays on different sized chips and they are ready to go.
Q. The technology uses nanolitres instead of the typical microlitres volumes used in molecular biology reactions. How is this achieved?
The main reason which allows customers to reduce the amount of samples and reagents is really due to the microfluidics technology which is at the heart of our IFCs. By having hundreds of individual fluidic circuits that combine the different samples with the different assays, all on one chip, we are capable of reducing the volumes required to perform any type of molecular assay.
Q. How much can a lab save by moving to your nanolitre-based technology?
Quite a lot actually. Without taking into consideration the amount of money that can be recovered by actually automating the whole NGS library prep process (which of course frees up operator time), the use of our IFC technology can cut down costs by up to 2 or 3 times the amount that is spent on classical NGS library prep techniques.
Q. Is the quality of data compromised or affected by using nanolitre volumes instead of the typical microlitres?
Absolutely not, and that is really one of the strengths of the technology. It is actually by miniaturizing the assays and by using the inherent benefits present within the IFC's that customers can use a system that is incredibly consistent and reproducible since it avoids all types of negative assay interactions and cross-contamination
Q. What instrumentation does a lab need to perform this? Is there much training involved to use new devices? And what are the capital costs involved?
As mentioned previously, the Juno system is a bench-top system that can be easily installed and operated in any laboratory. Our users are trained and assisted by our expert team of Field Application Specialists and Field Engineers and can get up and going using this technology in no time.
It is also important to note that the total cost of ownership, but especially initial capital equipment costs to acquire the instrumentation are significantly lower compared to other larger instruments which typically are doing the same thing (like liquid handling robots).
Q. Why has Fluidigm invested so much time and effort in bringing IFCs into the molecular biology lab?
As mentioned, IFCs were really created with the idea of being capable of automating molecular biology assays in nanoliter volumes. For more than 20+ years Fluidigm has been capable, thanks to its IFCs, to allow its customers to achieve greater cost-effectiveness, scale sample throughput, and improve time management all by simply having our customers load their samples on our plates. And since none of the assays that we use are pre-spotted or fixed, this means that our customers have the possibility to be incredibly flexible in how they use our technology and adapt it to their every need.
Request Information using button provided below or visit Fluidigm.com to learn more and click here to watch a live recording explaining the workflow.
Biography