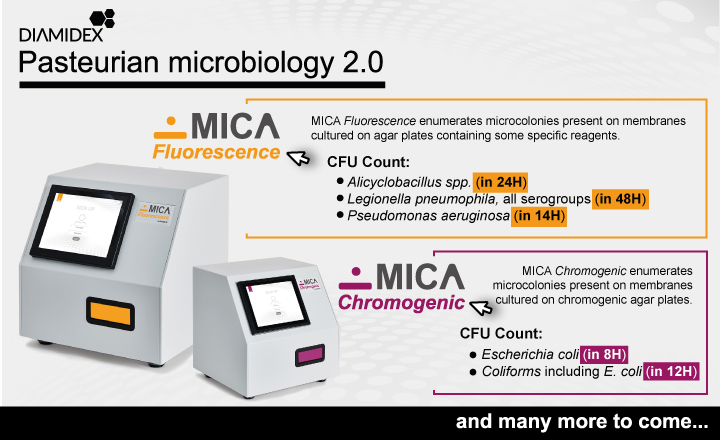
MICA Alicyclobacillus is a semi-automated solution for the rapid detection and counting of Alicyclobacillus in beverages. Based on patented technology, it provides automatic counting of Alicyclobacillus in 3 minutes, after just 24 hours of incubation on BAT agar plates.
While using culture and the same process as IFU No. 12:2019 standard method, MICA’s technology allows unmatched rapidity to deliver reliable results from 1 CFU per membrane. MICA Alicyclobacillus is designed specifically to improve productivity and responsiveness on stock rotation management for the beverage industry.
Process:
Same as IFU No. 12:2019 standard method, with one reagent added between membrane and agar.
After sampling, the test is a 3 steps process:
- Filtration of the sample after thermic shock, as in the traditional IFU No. 12:2019 standard culture method. The only difference is one reagent being added between the membrane and BAT agar.
- Incubation 24 hrs.
- Automatic 3 min detection and counting of Alicyclobacillus with MICA equipment and software.
The membrane could be re-incubated on BAT Agar for further identification or guaiacol test.
Key Points:
- Competitive price.
- Fast & reliable results in 24h allowing greater sensitivity and responsiveness
- Non-toxic procedure leading to the possibility of re-incubating the membrane on BAT Agar for further identification or guaiacol test
- Ergonomic & easy to use - sold with one-day training program
- Integration with LEAN management system: repeatability of results and readings
- 21 CFR Part 11 compliant
- Rights management per user, individual login
- Integration with usual management system, LIMS or CRM
For more information, please click here