Diagnostic microbial test providers will typically perform an experiment to detect specific pathogens in a food matrix, otherwise known as a method validation. The performance of the new method is typically compared to the traditional cultural bacteriological method for accuracy, sensitivity, selectivity, limit of detection and robustness to ensure the results are within the scope of its intended use. The goal of this process is to demonstrate the new method performs equivalent or superior to the reference method. This provides the user with the confidence the method selected for use meets or exceeds their food safety needs. But what if the food type you intend to test has not been validated or the method was not designed appropriately. How should you proceed?
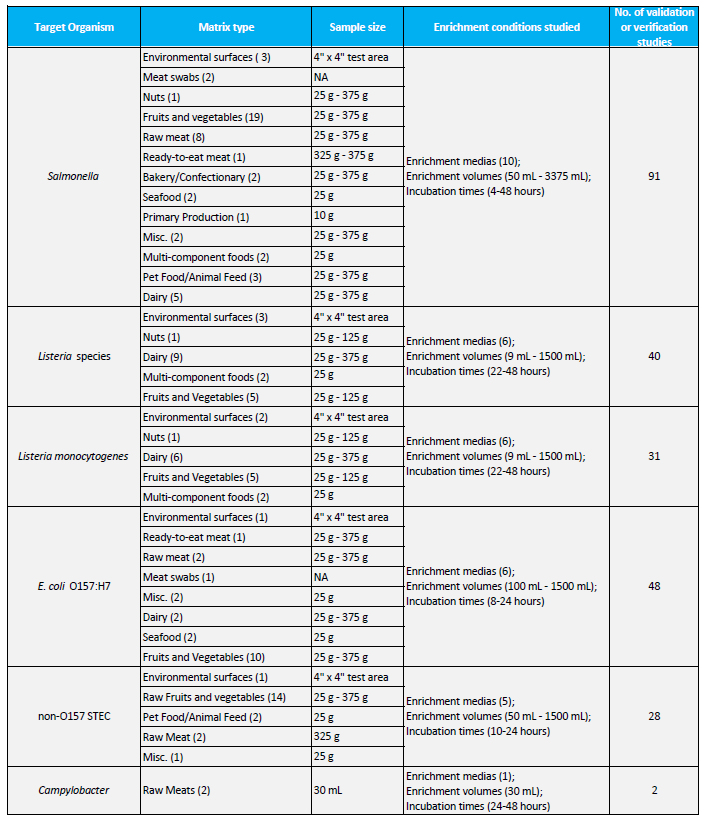
Hygiena has a dedicated internal applications team managed by Julie Weller focused on generating fit-for-use data for specific products and conditions. In addition to the numerous certifications granted to the BAX® System, this team has validated over 240 pathogen-matrix combinations under the same extensive study design required by certification bodies (Table 1). This unrivaled expertise enables Hygiena to provide exceptional support to its customers when it comes to matrices that have emerged as high risk or less studied.
Table 1
When an unknown matrix is under evaluation, preliminary tests should be performed prior to the validation phase to increase the probability of success. This can include a matrix assessment and determination of appropriate growth conditions (enrichment) of the target pathogen in the food matrix at varying levels of contamination.
Validations are not a “one-size-fits-all” and success depends on the understanding of the food matrix and the target pathogen. Matrices vary considerably both physically and chemically which presents numerous challenges for pathogens. Most foods are complex, composed of many different ingredients. They can contain antimicrobial compounds, polyphenols, fat and oils, large proteins; all of which can adversely affect recovery and detection. If there is a matrix effect on a pathogen, it can negatively impact the ability of the test method to detect it. The best way to assess this is by the use of an internal positive control (IPC) in every reaction which can validate the success of the test even in the absence of the target. This important feature can mitigate false negatives and ensure that the test would have accurately detected the pathogen if it were present.
Pathogen contamination is commonly present at low levels, sometimes as close to 1 cell per test portion and are often injured or stressed which can make recovery difficult. Statistically, the chances of detecting that 1 cell is far too low for a test to be accurate without an enrichment step. The primary purpose of the enrichment is to increase the number of healthy target bacteria to a detectable level whilst minimizing the number of natural non-target flora competing for the same nutrients. This is accomplished by combining the matrix with a liquid nutrient medium and incubating the sample at a specified temperature and time, optimal for supporting pathogen growth. For example, Salmonella and Listeria monocytogenes grow best between 35-37°C, but their growth rate or doubling times are quite different. Some healthy Salmonella can double every 20 minutes while L. monocytogenes takes between 45-60 minutes. These differences require samples to be incubated for different periods of time, 10 hours vs. 24-48 hours. Once optimized, a final enrichment protocol can be established.
The next step is to evaluate several contamination levels to determine how much of the target pathogen is needed to define the validation protocol. The inoculation level is typically low (0.2-2 CFU/test portion depending on the matrix) with the intent to demonstrate the detection limit of the method. This allows for the lowest concentration of the pathogen to be detected with acceptable certainty.
Many factors need to be built into a successful validation process as described above. It is imperative the microbial test method used in industry and regulatory meet the highest analytical performance standards for their intended use. The BAX® PCR system has been tested under stringent validation criteria to generate an extensive validation library supported by a dedicated applications teams. The decades of experience backed with exceptional confidence in results ensure the safety of the nation’s food supply.