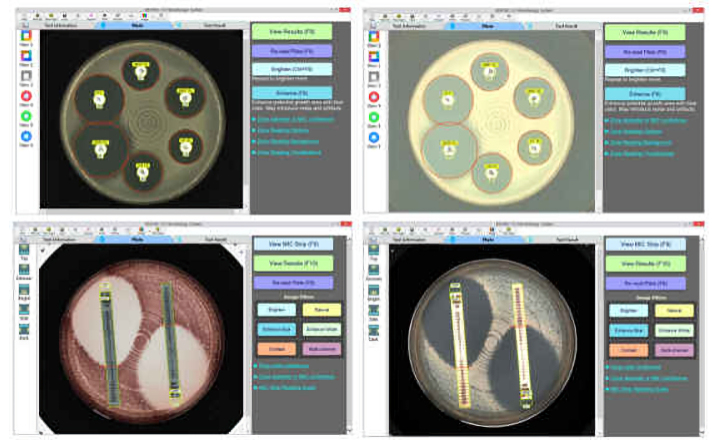
Sysmex Corporation has launched a testing system("the System") in Europe for rapid detection of antimicrobial susceptibility. The System detects the presence or absence of bacteria and assesses the effectiveness of antimicrobials using urine samples from patients suspected of having urinary tract infections (UTIs).
Using a unique and proprietary microfluidic technology, the System delivers antimicrobial susceptibility testing (AST) results in as little as 30 minutes from the start of measurement, a significant reduction compared to the several days required for conventional AST. The System not only promotes proper use of antimicrobial drugs in primary care settings, which are often the first point of contact for patients, but also contributes to the fight against antimicrobial resistance (AMR), a global challenge that requires concerted action.
Proper diagnosis and timely use of effective antimicrobials are of crucial importance in the treatment of bacterial infections, such as UTIs. In recent years, the COVID-19 pandemic has pushed clinical testing for infection diagnosis closer to patients, who often receive such testing at local clinics.
Unfortunately, it was not possible to prescribe antimicrobials based on the AST results during the initial patient encounter, as traditional AST methods can take several days to deliver results. This delay can contribute to the emergence of AMR. The emergence of AMR reduces patients’ access to effective antimicrobial treatments, which not only worsens their condition and reduces their quality of life (QOL) but also leads to serious economic consequences such as escalating medical costs due to prolonged hospital stays and the use of expensive drugs. This underscores the urgent global need to promote proper antimicrobial use in healthcare.5
The System, comprising a compact desktop-sized analyzer and sample cartridges, can automatically detect the presence or absence of bacteria in urine samples from patients suspected of having UTIs usually within 15 minutes and can perform AST to assess the effectiveness of various antimicrobials against any detected bacteria in as little as 30 minutes from the start of measurement.
This assists the appropriate use of antimicrobials during initial patient visits at clinics and other primary care settings. By employing a unique and proprietary microfluidic technology developed by Sysmex Astrego, which became an entirely owned subsidiary of Sysmex in May 2022, the System delivers significantly faster results in determining the effectiveness of antimicrobials than traditional AST methods, which can take several days to culture bacteria and assess their susceptibility to different drugs.
Furthermore, the System is user-friendly; directly placing a sample on the reagent cartridge and inserting the cartridge into the analyzer allows the device to automatically perform all the necessary measurements. It can be easily operated by healthcare professionals without specialized laboratory testing expertise.
The System will initially be introduced in the European market to raise awareness and gather evidence of its clinical usefulness before eventually expanding to other regions and broadening the range of applicable diseases.
This testing technology has the potential to revolutionize traditional clinical workflows for infectious diseases and address unmet medical needs for infection diagnosis. Sysmex is dedicated to tackling the global threat of AMR by making this innovative technology accessible to a wide range of primary care settings.