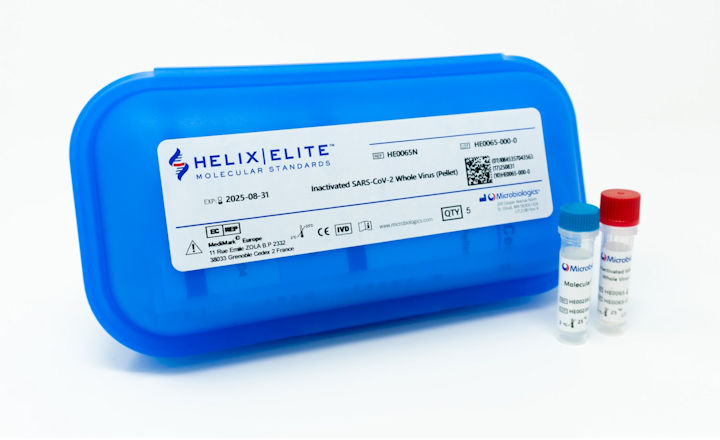
Microbiologics’ SARS-CoV-2 IVD controls bring much-needed simplicity in QC for the proliferation of assays with diverse gene targets. The Inactivated Whole Virus Controls provide the complete SARS-CoV-2 RNA genome and viral antigens in a non-infectious state confirmed by a validated assay.
The Process Controls contain a broad range of diagnostically relevant RNA sequences (Orf1ab/RdRP/S/E/ORF8/M/N gene regions) encapsulated in a phage protein envelope to validate the complete molecular assay procedure, including extraction.
Advantages:
- Lyophilized for stability and convenience, able to ship without dry ice
- Formatted to mimic patient samples to challenge sample collection and handling
- Mixed with a matrix of human epithelial lung cells to validate sample adequacy
These new IVD controls challenge each step in a molecular assay procedure, from sample collection to PCR signal detection. Available in pellet and swab formats, these controls join the most comprehensive portfolio of SARS-CoV-2 standards for quality control and research.
These new products join an extensive molecular portfolio that includes controls for Influenza. In addition to molecular standards, Microbiologics also provides custom BSL-2 and BSL-3 virology services to support research and development for SARS-CoV-2, Influenza, and other viruses.