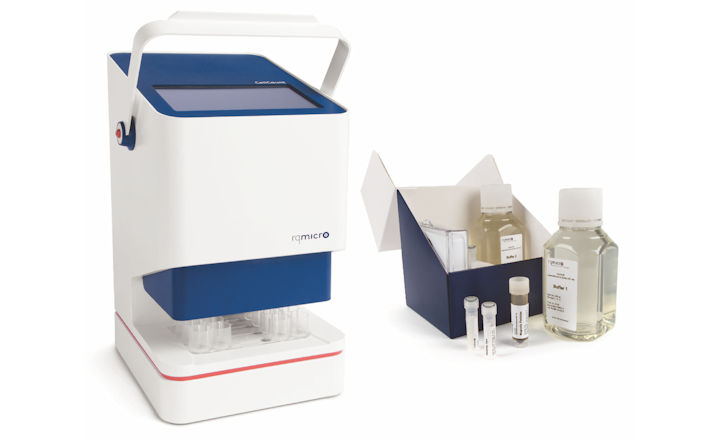
Currently, reliable quantification of Legionella is not possible. To overcome this problem, we have addressed several issues that occur with current analysis methods.In our opinion, automated and standardized sample preparation is imperative. Any sample preparation step, that could have an influence on the state of Legionella, has to be avoided. Therefore, a system is required that allows frequent on-site analysis to prevent misleading effects during sample transport. Finally, accurate and reliable discrimination between live and dead Legionella, including VBNC, is necessary.
For the above reasons, the rqmicro.COUNT instrument, together with the rqmicro reagents, were developed. It allows the quantification of intact Legionella pneumophila SG1-15, including VBNC, within 2 hours on-site. The rqmicro.COUNT is portable, robust and easy to use. Standardized sample preparation and analysis are performed automatically in a disposable cartridge for four samples in parallel.
In the first step, Legionella cells are purified by immunomagnetic separation, followed by single-cell analysis to determine the number of intact Legionella cells. The instrument is controlled through an intuitive software, which guides the user in a few steps through predefined analysis programs.
The instrument needs no manual adjustments or alignments and is maintenance-free. In this way, it offers a perfect solution for monitoring Legionella in various water systems and provides real-time information to optimize treatment protocols and mitigate risk.
The rqmicro.COUNT will be launched in summer 2020. Furthermore, the method can be adapted according to the needs of labs, which have already an analysis instrument, e.g. a flow cytometer, or need very high sample throughput.