To prove the accuracy of their results, pharmaceutical laboratories may participate in Proficiency Testing (PT) programs.
A PT program or an Interlaboratory Comparison is the organization, execution, and evaluation of measurements or tests carried out on the same or similar item by several laboratories in accordance with predetermined conditions.
These external quality controls enable laboratories to:
- Evaluate the accuracy of their analytical results,
- Control and improve the performance of their analysis,
- Check the good functioning of their equipment as well as the technical skills of their staff,
- Giving their clients confidence regarding the quality and safety of their products or services,
- Promote their analytical performance to their clients.
For the pharma industry, BIPEA offers three PT programs:
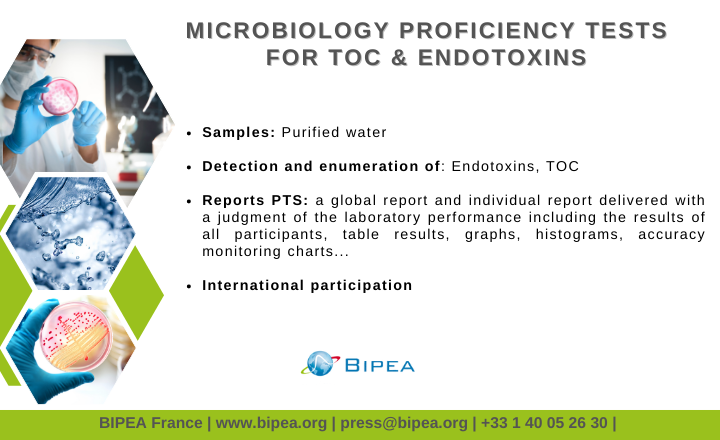
- PT 97 Purified water for pharmaceutical applications: Endotoxins and TOC in purified water.
- PT 98 Residual solvents in excipient: Ethylene oxide and Dioxane in Polyethylene glycol.
- PT 104 Microbiology of pharmaceutical process water: total viable aerobic count in water.
These three programs are designed according to the pharmacopeia requirements (European or others).
Details of the TOC and Endotoxins program:
- Proficiency testing scheme created in 2020
- 17 registered laboratories from 10 countries
- 2 rounds per annual series
- The time for analysis is 4 weeks
- Samples are shipped via express carrier.