International pharmacopeia stipulate a 14 day sterility test period. ATMPs may have a shelf-life of less than 48 hours. Traditional methods cannot be used for release testing of ATMPs prior to treatment. The risk of administering potentially contaminated ATMPs to patients urges both industry and authority to find solutions for rapid release testing.
Sartorius cares for patient safety. After our validated Mycoplasma Detection Kit, we now release your next support: Microsart ® ATMP Bacteria.
- Total bacteria detection within 3 hrs (Real Time PCR)
- qPCR Kit validation based on EP 5.1.6., EP 2.6.27, USP<1223>
- Sensitivity proven for 21 selected bacterial species including 4 standard USP strains
- Sensitivity proven for 17 critical cell therapy contaminants that are difficult to detect by classical sterility testing
- Lyophilized reagents - Stable reagents with constant high quality
- Specific TaqMan® probes reduce false-positives
- Non-infectious validation standards
- Less pipetting: controls already included
- All suitable qPCR cycler can be used
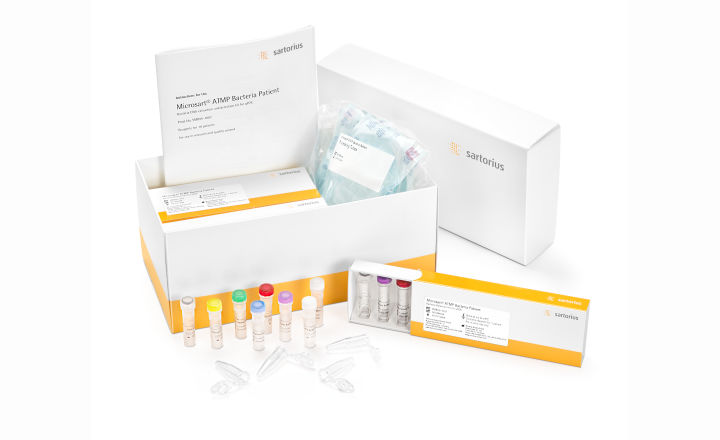
The validated Patient Kit will come ready-packed to test 10 individual cell therapy products for patients. For every individual ATMP to be tested, all solutions necessary to extract DNA and detect for bacterial contamination are packed into one separate box. This will avoid cross contamination during PCR pipetting, supporting your strong confidence into the ATMP you provide to help patients.
We are proud to support health.