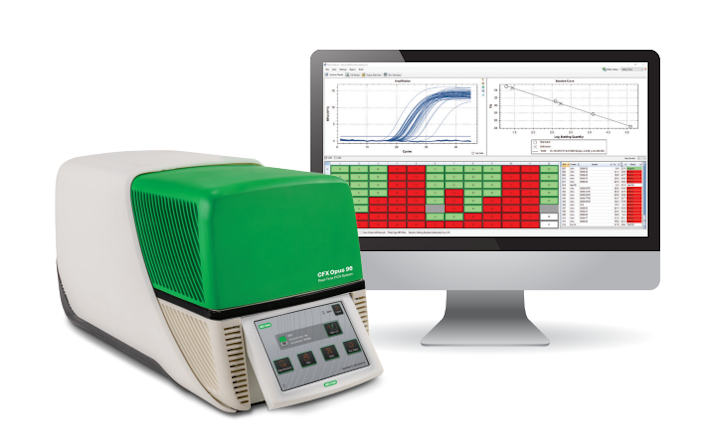
Bio-Rad has announced that AOAC INTERNATIONAL and AFNOR have approved the CFX Opus Deepwell Real-Time PCR System for use with Bio-Rad iQ-Check Real-Time PCR Kits.
AOAC INTERNATIONAL has approved the thermal cycler for use with all iQ-Check kits. To obtain approval, a validation study was performed, as part of the AOAC Performance Tested Methods Program, that demonstrated no differences in results between the iQ-Check and reference methods.
AFNOR has approved the instrument for use with the iQ-Check Salmonella spp., Listeria spp., L. monocytogenes, E. coli O157:H7, and Cronobacter spp. PCR Kits. The validation follows the ISO 16140-2:2016 standard and AFNOR technical rules.
The CFX Opus Deepwell Real-Time PCR System offers a modern user experience with flexible connectivity options, high-performance thermal uniformity, and cloud connection capabilities while maintaining the same performance standards customers have come to trust. The certificates are available at www.bio-rad.com/validations.
For more information on Bio-Rad’s complete range of iQ-Check real-time PCR test kits, click here or use the green "Request Information" button below to ask for details.