The addition of the PyroWave® XM Fluorescent Reader onto the PyroTec® PRO Robotic Endotoxin Testing System expands the capabilities of the PyroTec PRO System from solely kinetic LAL assay capabilities, with the PYROGENT®-5000 Turbidimetric LAL Assay and Kinetic QCL® Chromogenic Assay, to include the PyroGene® rFC Assay.
With one or two absorbance readers and a fluorescent reader, the PyroTec PRO Automated System can easily accommodate different assay types in the same run. Powered by the world-class WinKQCL® Endotoxin Analysis Software, this complete system helps ensure data integrity from automated endotoxin testing.
Data presented at the 2021 PDA Pharmaceutical Microbiology Conference demonstrate that automated endotoxin testing with the PyroTec PRO System produces results similar to manual methods with all three endotoxin test types, and reduces variability due to human error.
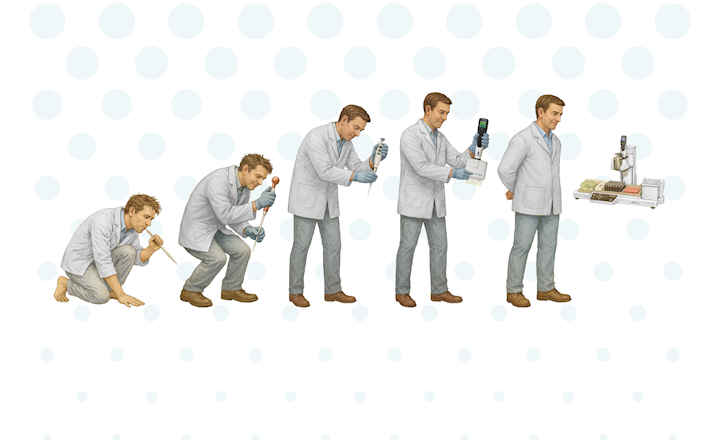
Achieve the error-reducing benefits of automation while preparing for the future with the PyroTec PRO System. Using the sustainable PyroGene rFC assay on the PyroTec Pro System your lab can reach 3R (Reduce, Reuse, Recycle) principles, without giving up traditional LAL assay capability. Training and data analyses are streamlined with WinKQCL Software integration across all test types. Lab personnel are freed from mundane, repetitive tasks, allowing more focus on higher-value activities such as data analyses.
Visit www.lonza.com/endotoxin-automation or use the green "Request Information" button below.