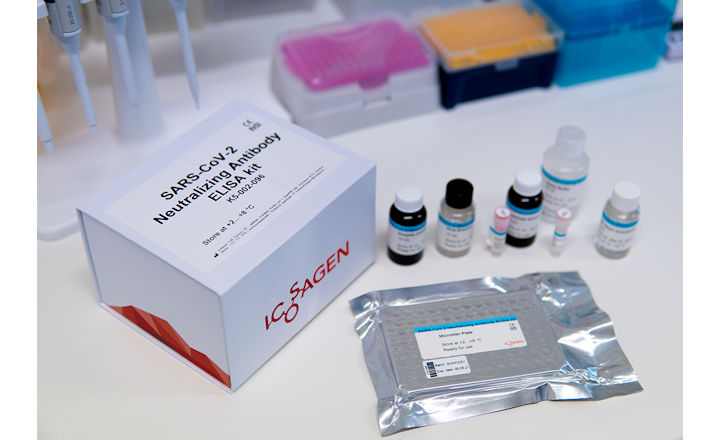
The SARS-CoV-2 neutralizing antibody ELISA test provides a qualitative in vitro determination of neutralizing antibodies of SARS-CoV-2 in human serum or plasma to either characterize obtained protective condition from previous encounter with the virus or the developed immune response after vaccination.
Because it is possible that COVID-19 disease and SARS-CoV-2 vaccines may induce antiviral immunity only for a limited period of time, the SARS-CoV-2 Neutralizing Antibody ELISA kit allows to determine the duration and efficacy of antibody immunity. In addition, the test allows medical facilities to select highly neutralizing blood serums of patients who have recovered from COVID-19 disease, which then can be used to treat critically ill patients.
Icosagen's SARS-CoV-2 Neutralizing Antibody ELISA kit has already played a key role in the development of our BioBlock® nasal spray, which has been developed in collaboration with Estonian researchers and entrepreneurs. BioBlock® is a natural formulation derived from bovine colostrum and contains bovine antibodies to SARS-CoV-2 virus.
The current ELISA test will be further developed to detect the presence of protective antibodies against other rapidly spreading and severe SARS-CoV-2 strains, such as the UK (B.1.1.7), South African (B.1.351), Brazilian (P.1), and Indian variants.
Recent studies have shown that antibodies to common virus strains currently present in Europe offer less protection against newer strains, especially to Brazilian and South African variants. Thus, from the vaccination strategy and epidemiological point of view, it is important to know which strains the Estonian population has protection and which strains carry a higher risk of disease.
Icosagen Cell Factory OÜ's SARS-CoV-2 Neutralizing Antibody ELISA kit was registered on 16 April 2021. With this, Icosagen has already launched another complete CE-IVD product (CE-marked in vitro diagnostic medical device) in Estonia.
The test is intended for professional use only, as laboratory training personnel and special laboratory equipment are required. The development of diagnostics was supported by the Government of Estonia with €100,000 grant.
Request information using button provided below or visit Icosagen.com to learn more.
Note: This content has been edited by a rapidmicrobiology staff writer for style and content.