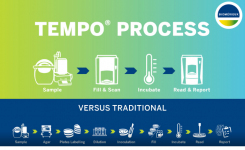
Endotoxins (also called pyrogens) are found in the cell walls of Gram -ve bacteria, they are heat stable toxins that can affect mammals even in small doses. The most commonly used approach now is a Limulus Amoebocyte Lysate or LAL test.
This guide to endotoxin testing reviews the various techniques for the Bacterial Endotoxin Test or BET together with a list of suppliers of LAL Gel-Clot reagents, LAL Chromogenic reagents, LAL Turbidimetric reagents, Pyrogen-free laboratory disposables and instrumentation for high through put such as contract testing service laboratories.
Click here to visit Guide to Endotoxin Detection Methods.