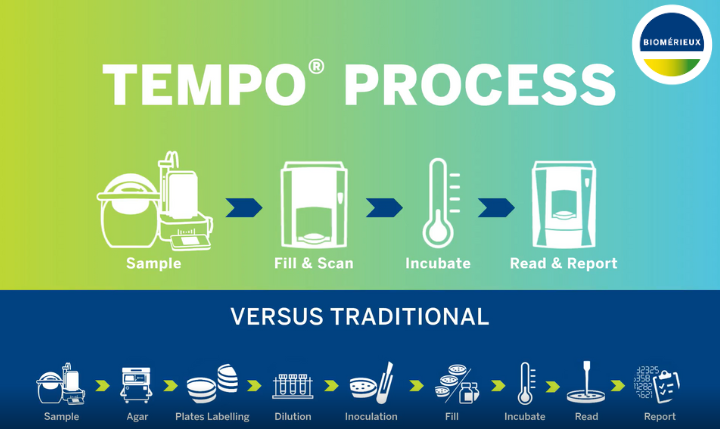
PathogenDx, Inc., has announced that its QuantX Total Yeast and Mold test, the world's first quantitative microarray test for cannabis, has received an AOAC® Performance Tested Methods SM (PTM) Certificate from the AOAC Research Institute, making PathogenDx the first and only molecular technology company to receive such an AOAC certification. The news coincides with the launch of its updated suite of innovative cannabis testing solutions that advances safety and quality standards in the industry.
Designed for the cannabis and hemp industries, PathogenDx's QuantX assay quantifies the total amount of yeast and mold in a sample while also determining if the sample exceeds industry testing and safety standards. The test provides results in less than 6 hours compared to 3 to 4 days for conventional culture or enrichment-based methods. QuantX is suitable not only for cannabis testing labs, but also for growers, whose product must adhere to a limited amount of yeast and mold contamination.
Leveraging its learnings from the agile development of COVID-19 diagnostics, PathogenDx has innovated upon its multiplexed microarray testing platform and introduced an expanded 96-well plate, improved sample preparation and streamlined data reporting with the introduction of a new, customized portal. PathogenDx also released its Detectx--Alive test that separates live cells from dead cells for cannabis bacterial or fungal safety tests to provide increased accuracy, assurance and cost-savings. These updates bring the cannabis testing industry up to the same level of standards used in the food, agriculture and human diagnostics sectors.
"We are proud that our QuantX microarray has achieved the highest level of AOAC certification, just in time for the release of our new suite of advanced testing and reporting technologies that raise cannabis testing closer to the level of efficacy and standardization required of labs in mainstream industries," said Milan Patel, Co-founder and CEO of PathogenDx. "We implemented our nuanced learnings from our work combating the COVID-19 pandemic to drive efficiency, cost-savings, improved results and greater compliance for the cannabis industry. As the sector enters an era of acceptance at a national level, PathogenDx is committed to ensuring that the methods used in cannabis testing are not home-grown but standardized methods recognized at the federal level. We most notably appreciate the leadership that the AOAC organization has taken on this front to support the industry. With these developments, cannabis testing labs will now mirror how labs in other industries have been operating for decades."
Expanded 96-Well Microarray
PathogenDx expanded its 12-well Microarray into a standard 96-well format, introducing to the cannabis industry a best practice commonly used in clinical labs. Made with superior-quality glass, the larger format offers an improved level of efficiency, specificity, imaging accuracy and economies of scale. PathogenDx is also releasing an industry-first with foil-sealed wells. The updated plates enable lab technicians to remove the foil only on the wells needed for samples received for testing that day or shift, realizing significant cost savings from reduced waste of unused wells and test media.
Streamlined Processing on Quantx
PathogenDx introduced a one-step PCR for its Quantx fungal assay via a sample preparation step using a simplified spin-column step. The new methodology for preparing and analyzing cannabis matrices improves assay reliability by reducing PCR inhibition and minimizing all types of dim signal. This step also shortens the process by consolidating the two-step PCR into a single PCR step, enabling results to be delivered in 4.5 hours instead of 6 hours.
Detectx-Alive
Testing accuracy is jeopardized when the treatment of inputs results in cell death and an environment wherein both live and dead microbes exist. To address the concern of false positives, PathogenDx developed its Detectx-Alive test, which isolates live DNA from the deceased cells in any bacterial or fungal safety test and delivers results in 6 hours or less. With increased accuracy, testing labs save expenses by removing the need for expensive re-tests and avoiding unwanted product losses.
PathogenDx Portal
To provide another level of granularity in test results reporting, PathogenDx is replacing DropBox with a custom PathogenDx Reporting Portal for cannabis compliance reporting. The intuitive, user-friendly portal drives customer ease and efficiency by reducing the number of steps necessary to obtain lab results and COAs. It also improves data visibility with multi-user access to real-time results tracking and prior history reports. PathogenDx plans to add new integrations, all product inserts and ordering capabilities for PathogenDx products in the next 12 months.
Visit pathogendx.com
Note: This content has been edited by a rapidmicrobiology staff writer for style and content.