BioSys Gains AOAC Approval
go back to news archives
| BioSys have recently gained approval for the testing of coliforms in a range of dairy products. |
| 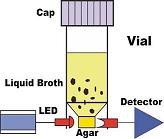 |
Source : BioSys, Inc. [USA]
Now part of Neogen Europe Limited View Company Information
Posted on August 11, 2003
LATEST MICROBIOLOGY NEWS
MICROBIOLOGY EVENTS
-
Unmasking Endotoxins: A Sample Preparation Strategy to Overcome LER
15 Jul 2025 -
Next-Generation MAT vs. Traditional Pyrogen Tests: Faster, Ethical, and More Reliable
15 Jul 2025 -
LIMS Launchpad Series: Episode #1 - Strategic Planning
17 Jul 2025 -
IAFP 2025
27 Jul 2025 -
ADLM 2025
27 Jul 2025 -
Food Safety Culture Workshop
9 Sep 2025 -
Who are the "Real" Spoilers in Food?
11 Sep 2025 -
CPD accredited course: Level 3 HACCP & Food Safety
15 Sep 2025 -
Culture Eats HACCP for Breakfast (free)
On-demand Webinar -
A3P International Congress 2025
7 Oct 2025

















