The New Technology Showcase at this year's IBMS congress in Birmingham, UK (28th - 30th Sept., 2015) will include a presentation by Dr Monika Stuczen, Laboratory Manager, Medical Wire & Equipment Co. Dr. Stuczen will be explaining the CSLI M40-A2 standard, which is the only standard for evaluating the performance of Swab Transport Systems in terms of bacteria recovery during transportation.
There are no other existing standards up to date. The standard provides criteria for the manufacturers and end users of transport devices assisting in testing and choosing an appropriate product for the transport of clinical specimens in order to provide the best possible patient care. There are two standardised protocols for testing swabs included in the standard, which can be utilised by laboratories – qualitative roll plate method and quantitative elution method.
In clinical diagnostics, successful sampling and transportation of microorganisms to the lab is crucial for an accurate diagnosis. Failure to recover microorganisms can have a dramatic impact on patient diagnosis and treatment. Therefore, end users should always choose transport devices, which are M40-A2 compliant.
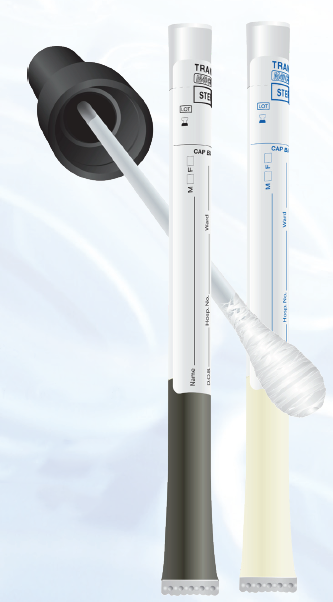
To be fully compliant with M40-A2 means that transport swabs are able to recover all aerobic, anaerobic and facultative bacteria defined within the standard at both 4ºC and room temperature. Also, transport swabs should be free from non viable microorganisms as the presence of such bacteria may lead to misdiagnosis.
The New Technology Showcases focus on some of the latest innovations and technology in the biomedical science industry. The talks include next generation technologies, new workflows and systems, transforming pathology and lab automation. All the talks are free and form part of the Exhibition at IBMS Congress 2015 which enables delegates to view new equipment and products, extend general awareness of techniques and technologies, and make contact with representatives of the exhibiting companies.
All Medical Wire Transwabs® are FULLY compliant with M40-A2 - to see what is required for compliance with CSLI M40-A2 click here or for more information please contact rseuke@MWE.co.uk