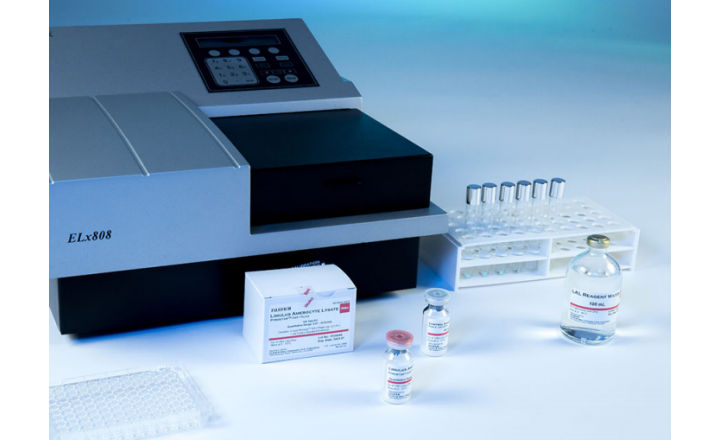
PYROSTAR™ ES-F/PLATE is a multi-test vial kit having the same endotoxin specific formulation as our other ES-F series products yet designed to be used with the microplate reader. To use this configuration, the user dispenses 0.05 mL of the sample into the microplate followed by 0.05 mL of dissolved LAL reagent. This assay is ideal for testing a larger number of samples in one run.
Key Features:
- Endotoxin-specific reagent avoids false-positive results from glucans
- 2.0 mL volume equates to approximately 40 tests per vial
- 5.2 mL volume equates to approximately 100 tests per vial
- The sensitivity ranges from 0.01 to 10 EU/mL