The global aseptic food and beverage packaging market is expected to reach $35 billion by 2025, supported by the increased popularity of dairy. The dairy-alternatives market is expected to reach $53 billion by 2028, currently being led by soy-based products with almond, coconut, and oat milk gaining popularity.
‘Milk’ products are routinely processed by thermal methods such as pasteurization (done for decades). Drawbacks to these methods are restricted or limited shelf life, chilled distribution, and refrigeration after purchase. Increased demand has forced manufacturers to evaluate aseptic methods for processing and packaging these products. Complicating this are increased costs, increased holding of inventory until product release, and the need for more technically specialized staff. To offset these costs, manufacturers are looking for ways to streamline processes, including testing final products for contamination.
For contamination testing, traditional testing uses direct and indirect methods to detect microorganisms in finished products. While these processes are relatively reliable, they are manual, error-prone processes which can take up to 15 days for results, consuming valuable resources, time and incubator space. Other tests, such as pH measurements, also require follow-up tests to confirm microbial contamination (i.e., plate culture).
The problems with visual inspections of cultures are many; numerous plates must be carefully evaluated, even if no growth is initially seen to confirm no small colonies are present. In addition, colony growth on a plate does not identify the organism – further testing is required to classify and confirm the species, introducing the risk of errors and taking additional days to complete.
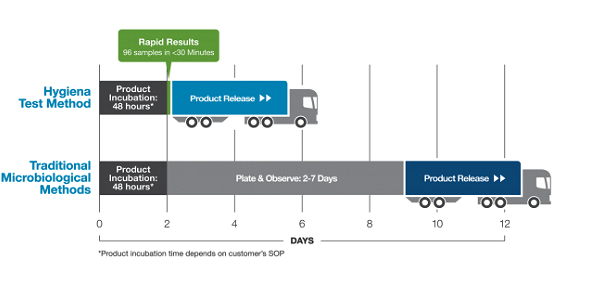
To reduce time to results and streamline laboratory testing, a rapid method is needed - Hygiena’s Innovate System. Results can be obtained in less than 30 minutes and up to 96 samples can be run at once (no secondary incubation needed as for other growth-based methods). In addition, supported product matrices are diverse – from milks and creams to sauces, soups, broths, juices and beverages. This is possible due to advanced technology that eliminates somatic cell components (including non-microbial ATP) prior to detection of microbial ATP. Also, the technology can be used across a wide pH range due to its high buffering capacity.
Following product analysis (<30 minutes), a product report is generated for all samples tested, allowing the rapid release of products testing negative. This allows for quick inventory reduction, allowing products to be shipped quicker. Overall, it decreases manufacturing costs – releasing product sooner means more revenue sooner, and faster movement of more product through manufacturing means more volume released at the same or less cost.
To fully validate this testing process, Hygiena™ recently worked with several dairy and dairy-alternative partners to test time to results for various beverages: UHT milk, ESL milk, oat milk and soy milk. The goal was to determine if the Innovate system could detect low levels of microorganisms in a variety of beverages using the RapiScreen™ Dairy kit for ATP detection, and comparing results to other methods, including pH and plating. Typically, a product is incubated in its own packaging to enrich the ATP from any contaminating microbial cells. As negative controls, uncontaminated products were used to establish baselines for comparison to determine what constitutes a positive result.
In all cases, for all organisms tested (except for Clostridium sporogenes in ESL oat milk), the Innovate System was able to detect low spike levels (~10 CFU per pack) at the 24 hour mark. RLU values in the spiked products exceeded the uncontaminated product RLU thresholds, set as three times the baseline values. Download the studies here:
- Rapid Detection of Microorganisms in Oat Milk Products Using the Innovate System
- Rapid Detection of Microorganisms in Soy Milk Products Using the Innovate System
- Rapid Detection of Microorganisms in ESL Milk Products Using the Innovate System
- Rapid Detection of Microorganisms in UHT Milk Products Using the Innovate System
As a result of rapid detection of low levels of contamination at 24 hours, the cost savings for any facility is significant. While QC costs are similar, the investment in finished goods and safety stock is significantly reduced along with the warehouse space costs. In addition, positive release of product helps prevent recall cost and protect the brand that adds significant benefit to the introduction of a rapid testing system such as the Innovate System. As an additional advantage, high-volume facilities can automate product sampling using the Innovate Autosampler III.
Learn more about the Innovate System and the Autosampler III here.

























