Advanced Therapeutic Medicinal Products (ATMPs) include gene, cellular and modified tissue products. Both starting materials and final infused or implanted products are living and therefore cannot be sterilised nor defined as sterile. This adds complexity to many aspects of risk management and patient safety, as well as GMP compliance.
Collection and processing of human starting materials through to final dose preparation involves multiple aseptic transfers and manipulations, all of which must be monitored and controlled. They also must be capable of being simulated with media fills for validation.
The development of completely enclosed single-use processing systems can reduce the risk of contamination in those processes. However, all equipment has a risk of failure, such as leaks, and an aseptic environment is still required. The patient’s condition and therapy often dictate that there is no opportunity for repeating the process.
Individual ATMP products and intermediates have specific requirements for removal and testing for antimicrobials, virus, specific microorganisms, endotoxins, etc. but they all share the GMP requirements for protection from environmental contamination. These include continuous monitoring for viable and non-viable particles and process validation with media fill simulations.
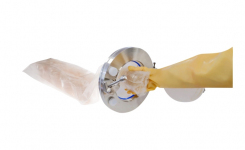
Cherwell Laboratories has many years of experience in packaging and irradiating agar plates to deliver sterile media into critical environments. The development and growth of ATMP production require more novel single-use systems and Cherwell offers bespoke aseptic filling of devices with culture media, often in low numbers, which cannot be achieved at the ATMP manufacturing site.
The latest Grade A compatible 'slit to agar' air samplers such as the ImpactAir ISO-90 enables enclosed continuous sampling for 4 hours onto a single rotation of a standard 90mm agar Petri dish to satisfy another of the revised Annex 1 requirements.