An independent study carried out by a World Health Organization (WHO) initiative to verify the specificity and sensitivity on COVID-19 molecular test kits has scored KH Medical's RADI RT-PCR Kit (Korea Republic) with the highest rating, achieving 100% in both categories.
It is the first round of studies performed by WHO collaboration partner, Foundation for Innovative New Diagnostics (FIND), and was done at the Hôpitaux Universitaires Genève, in Geneva Switzerland, where both WHO and FIND have their headquarters.
FIND is curating a global diagnostic pipeline and tracking reported global diagnostic use, and will be performing these independent studies on antibody tests in due course.
To be part of the study, FIND invited manufacturers around the world to participate and have their kits validated to create a centralized database of test performances 'as a global public good'.
These molecular test kit studies verify the limit of detection (LOD) and the clinical performance as reported by the manufacturers. The LOD analysis is performed by using cultured viral stocks from a clinical isolate from Switzerland and quantified using an E gene standard. The clinical performance analysis is conducted on extracted samples from individuals suspected to have COVID-19 that were tested using an in-house PCR protocol that was optimized based on the Tib Molbiol assay (WHO protocol).
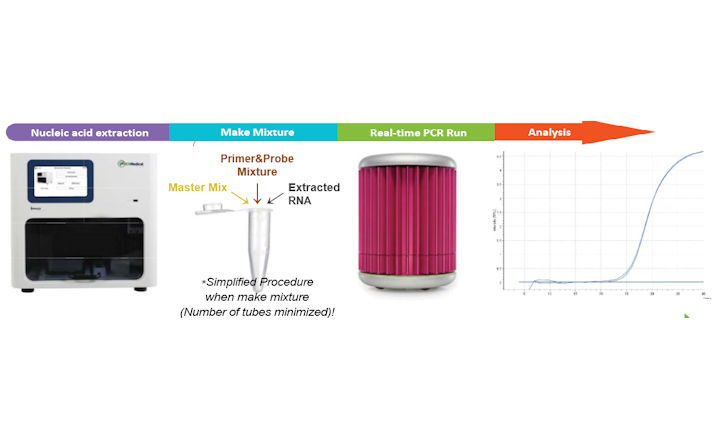
The RADI COVID-19 Real-Time PCR Detection Kit has CE-IVD marking and is used for the qualitative detection of SARS-CoV-2 in human nasal swab or sputum by using RADI Prep Plus (fully automated extractor) and RADI Real-Time PCR system and runs on all major thermocyclers, i.e. ABI 7500 or Bio-Rad CFX 96 Platform.
The assay has two targets as part of its assay for SARS-CoV-2; the S-gene (spike glycoprotein) and the RdRP gene (RNA-dependent polymerase). It has a limit of detection (LOD) of 0.66 copies/µL and can provide a sample-to-result turnaround in 80 mins.