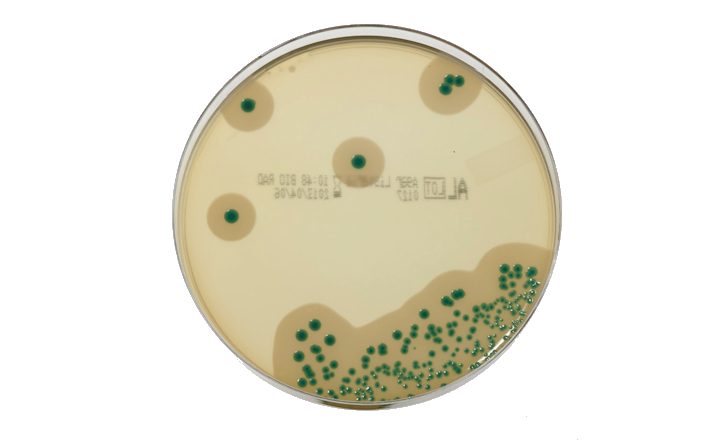
The Bio-Rad AL method NF Validation (ISO 16140-2) certification has been extended for Listeria monocytogenes and Listeria genus detection with a shortened enrichment time. This method includes enrichment step in Half Fraser broth, screening step on AL agar and in case of typical colonies, a confirmation step with various rapid tests. Now results can be obtained from 40 hours with the AL method for L. monocytogenes and Listeria genus detection.
Thanks to the high quality of Bio-Rad media’s, the new validated protocol of AL method offers a high level of performance with an enrichment from 18 to 28 hours at 30°C in Half Fraser broth. This improvement of protocol presents many advantages.
The reduction of the minimum enrichment time from 22 to 18 hours allows to reduce the final time to results and save at least a half-day for the batch release.
The larger enrichment incubation time range gives a flexibility of 10 hours to perform the next step of the protocol, which improves the laboratory routine organization for a better staff resource optimization. Furthermore, in case of unplanned staff resources changes, the laboratory organization could be adjusted thanks to the large time range to perform the screening inoculation step.
Often decreasing the enrichment time requires a higher incubation temperature, however, due to the high performance of Bio-Rad's media, this is not necessary. By keeping the incubation temperature at 30°C the laboratory can reduce the energy consumption needed to run the incubator.
The new validated AL method offers a better time to result, optimized laboratory organization, and an energy consumption saving protocol.
To learn more visit Bio-Rad or ask for details using the green "Request Information" button below.