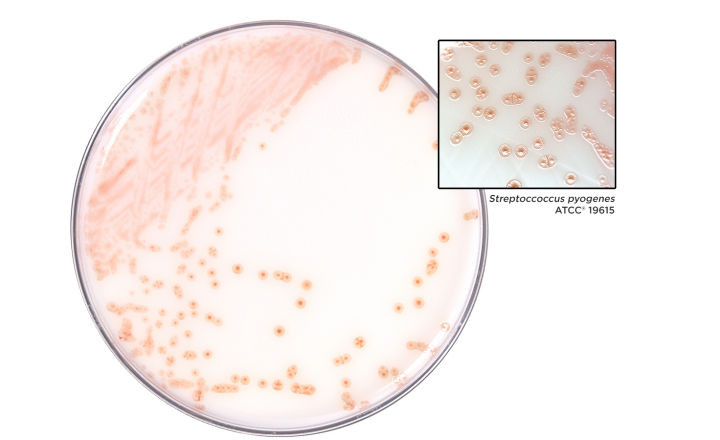
The recent reports of invasive Group A Streptococcal infections in children have drawn attention to this relatively common pathogen. Perhaps best known as a cause of pharyngitis, Streptococcus pyogenes is ubiquitous in the population, with many people being colonized without symptoms. It is, however, an opportunist pathogen, and if untreated, can cause more serious, potentially life-threatening illness such as scarlet fever, rheumatic fever, and necrotizing fasciitis.
Streptococcus pyogenes is readily diagnosed by microbiological methods, whether conventional culture, rapid direct tests or most molecular platforms. Swabs are taken from the patient’s throat or from skin lesions. It is important to use only transport swabs which are M40-A2 compliant[1]. Streptococcus pyogenes is one of the ten bacterial species included in the M40-A2 panel of test organisms against which compliant products have been tested.
MWE’s Transwab® with Plain Amies (MW170), Charcoal Amies (MW171), and Σ Transwab® with Liquid Amies (MW176S) are routinely tested for the effective recovery of Streptococcus pyogenes. In addition, a recent study demonstrated that the organism is successfully identified when tested on the Cepheid GeneXpert® XVI system utilising Xpert ® Xpress Strep A (XPRSTREPA CE 10).[2] Our products meet M40-A2 compliance.
We would be happy to help and support laboratories and kit manufacturers where needed. Our products have been used globally on various platforms, and we have the clinical evidence to back that. If you need further information, please click here or get in touch using the green "Request Information" button below.
References- Clinical and Laboratory Standards Institute (CLSI). Quality Control of Microbiological Transport Systems; Approved Standard- Second Edition. CLSI document M40-A2
- Laughlin, J., 2019, Molecular efficacy of two commercially available liquid transport collection devices for downstream testing on the GeneXpert® platform and the Roche FLOW® system, European Meeting on Molecular Diagnostics, 11th Meeting, Noordwijk, Netherlands