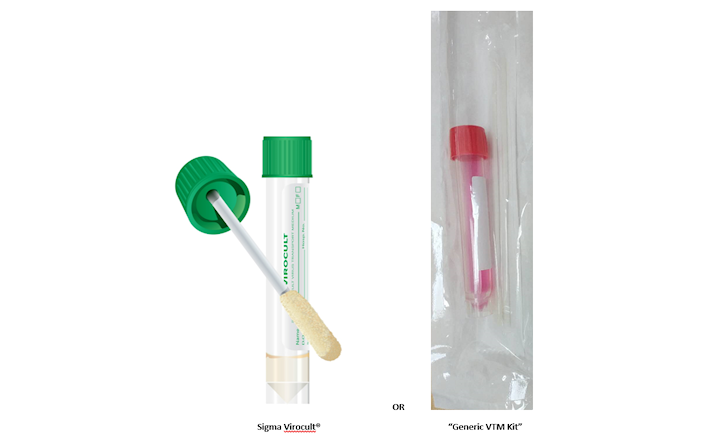
In these days of unprecedented demands for testing for COVID-19, many new and unheard-of “brands” are appearing, not just on social media, but turning up in specimen bags at laboratory receptions. It seems all that is required is a tube of generic red liquid and a swab of completely uncertain provenance.
All our years of attention to accredited performance and medical device safety have been thrown out the window in the relentless bid for “testing”. But will anything do? There has also been considerable use of alternative transport devices using non-virus specific media and even saline.
Testing for coronavirus is carried out on state-of-the-art molecular platforms using expensive reagents, and with highly skilled laboratory personnel, so can it be justified to use inferior collection devices.
MWE’s Virocult® medium has been around for well over 40 years. It has been shown in many studies to be completely reliable on molecular platforms, whether PCR or antigen, as well as the gold standard cell culture methods.
The proprietary formulation is our own, not just copied off of a website, using carefully selected ingredients of the highest possible purity, with long experience of developing the best methods for preparation, ensuring high sensitivity and specificity in all cases.
A recent study has shown that it could be possible to test directly from Sigma Virocult® using RT-qPCR without extraction, leading to significant savings in time and cost, with the possibility of scaling up testing capacity without compromise on performance or patient safety.
Other studies also demonstrate that Sigma Virocult® and its sister product Sigma Transwab® with liquid Amies can be used reliably on molecular platforms, while other products such as e-Swab® cause significant inhibition with much higher Ct values.
In recent years much effort has been expended by laboratories and reputable manufacturers in the development and implementation of quality standards, including European Medical Devices Directives (for authentic CE-marking), ISO 13485:2016, ISO 15189:2012, and CLSI M40-A2.
It is essential that in a time of crisis, we do not let our patients down by cutting corners and using inferior devices that give results that cannot be trusted.
References
- Smyrlaki, I., et al, 2020, Massive and rapid COVID-19 testing is feasible by extraction-free SARS-CoV-2 RT-qPCR, merRxiv preprint doi: https://doi.org/10.1101/2020.04.17.20067348
- Laughlin, J., 2019, Molecular efficacy of two commercially available liquid transport collection devices for downstream testing on the GeneXpert® platform and the Roche FLOW® system, European Meeting on Molecular Diagnostics, October 2019, Noordwijk, Netherlands
- Keeley A. J., et al, Roll-out of SARS-CoV-2 testing for healthcare workers at a large NHS Foundation Trust in the United Kingdom, March 2020. Euro Surveillance, 2020; 25(14):pii=2000433. https://doi.org/10.2807/1560-7917.ES.2020.25.14.2000433
- Lescure, Francois-Xavier, et al, 2020, Clinical and virological data of the first cases of COVID-19 in Europe: a case series, www.thelancet.com/infection Published online March 27, 2020, https://doi.org/10.1016/S1473-3099(20)30200-0
- Haigh, J., et al., 2019, A service evaluation of simultaneous near-patient testing for influenza, respiratory syncytial virus, Clostridium difficile and norovirus in a UK district general hospital, Journal of Hospital Infection 103 (2019) 441-446
- Bharucha, T., et al, 2018, Detection of Japanese Encephalitis Virus RNA in Human Throat Samples in Laos – A Pilot study www.nature.com/scientificreports (2018) 8:8018 | https://doi.org/10.1038/s41598-018-26333-4
- Salez, N., et al, 2013, Improved Sensitivity of the Novel Xpert Flu Test for Detection of Influenza B Virus, J. Clin. Microbiol. 51:4277-4278
- Johnson F. B., 1990, Transport of Viral Specimens, Clinical Microbiology Reviews,3:120 – 131
- Rudsdale, A., 2009, Evaluation Of A Virology Specimen Transport Device With Six Viruses Using CLSI Standard M40-A, Poster C-053 ASM 109th General Meeting, Philadelphia, 2009
- Council Directive 93/42/EEC of June 14 1993 concerning medical devices OJ L 169 of July 12 1993
- ISO 13485:2016 Medical devices — Quality management systems — Requirements for regulatory purposes
- ISO 15189:2012 Medical laboratories — Requirements for quality and competence
- M40 Quality Control of Microbiological Transport Systems, 2nd Edition, CLSI 950 West Valley Road, Suite 2500, Wayne, PA 19087, USA. www.clsi.org