The name Shenzen Bioeasy Technology is a familiar name to anyone who has been keeping up to date with developments in the COVID-19 diagnostic market. They were the first company to obtain CE-IVD marking for a COVID-19 Antigen test but were also developers of a COVID-19 test that was one of the first to be returned by laboratories for poor performance (Ag Colloidal test).
However, Shenzen Bioeasy's Flouresence Rapid Antigen Test remains the top performer for diagnostic accuracy through the WHO/FIND global network of laboratories who evaluate new tests on the market. Its evaluation was done in Santiago, Chile, by the Clinica Alemana de Santiago (Chilean-German Charitable Corporation), the leading evaluator of antigen tests through the WHO/FIND network.
The test which targets SARS-CoV-2's nucleocapsid protein demonstrated 93.9% sensitivity (picked up 77 positives from 82 RT-qPCR confirmed positives) and 100% specificity (proved negative for all 45 RT-qPCR confirmed negative samples). The test scored over 50% higher than most of its rivals in these evaluations.
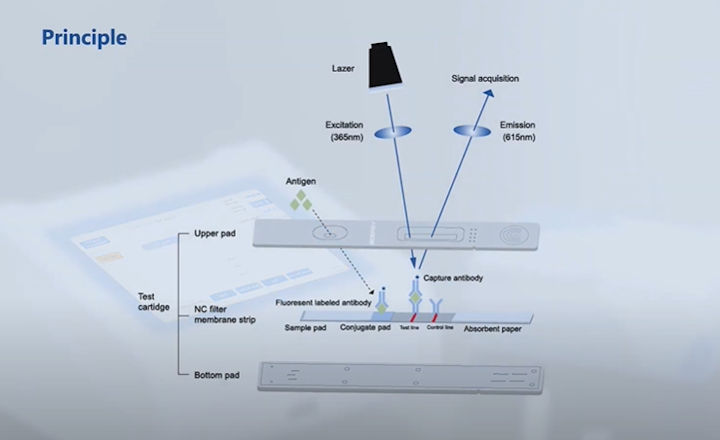
"The test runs on Bioeasy's Time-resolved Fluorescence Immunoassay Analyzer (TRFIA). TRFIA is a rapid, quantitative instrument that is used to test quantitative items such as cardiac markers inflammation, hormone, diabetes, etc. It uses time-resolved techniques to measure fluorescence, combines long decay lifetime markers with time-resolved fluorescence, effectively eliminating non-specific fluorescence interference and greatly improving analytical sensitivity." (Source: Bioeasy)
Watch a demonstration of its simplicity below.