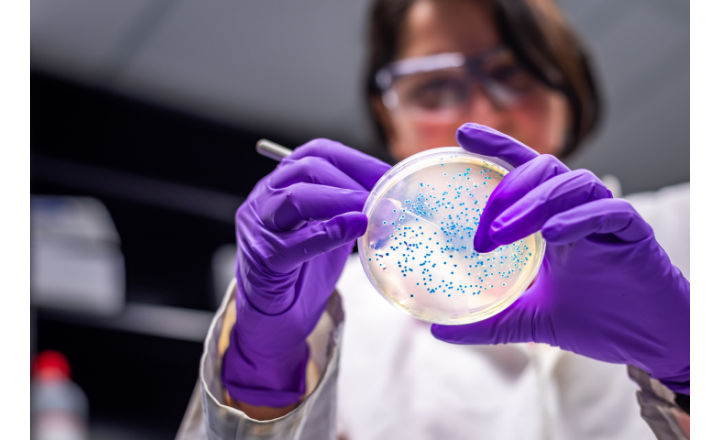
Bio-Rad has obtained ISO 17025 accreditation, from Cofrac, of its quality control laboratory in the manufacturing plant in Steenvoorde, France (N° 1-6642)
Louis-Marie Roque, Cofrac auditor, and independent consultant, and Jean-Michel Plancq, Bio-Rad Quality Manager, explain how this approach was implemented, the challenges encountered and the benefits for customers using Bio-Rad methods in an interview with Yannick Bichot, Business Unit Marketing Manager, Food Science Division, Bio-Rad Laboratories.
Questions to Louis- Marie Rocque:
Q: You recently accompanied Bio‑Rad in its ISO 17025 accreditation process. What was your role?
My role was to help Bio-Rad in its accreditation process according to the ISO 17025 standard, more specifically for the technical part. The culture media preparation unit was already operating with rigor; the work consisted of resuming the processes to comply with those of ISO 17025.
Q: Can you describe how ISO 17025 applies to a method provider in water and food microbiology?
Accreditation is recognition of the competence of a body to perform the services specified in its scope (of accreditation). For a manufacturer of a method in water and food microbiology testing, it allows its customers to guarantee the conformity of the controls carried out for batch release. It is a guarantee of seriousness and competence which is validated by independent experts.
Q: Do you consider ISO 17025 as a key accreditation for a manufacturer and do you think this standard is a ‘must-have’ for now and the coming years?
Quality control certificates issued by a manufacturer under ISO 17025 accreditation provide irrefutable proof that these controls are in conformity. In a world where customer requirements are becoming more important, being able to deliver products with quality control under accreditation is a guarantee of seriousness and confidence. Accreditation is recognized worldwide thanks to multilateral recognition agreements and will become an essential element in the future.
Q: What are the benefits for a laboratory to use an ISO 17025 accredited supplier?
Accreditation is a sign of trust. It allows a laboratory to trust its accredited subcontractor and to lighten its own internal laboratory controls. The ISO 17025 standard in its chapter 6.6 states that: ‘The laboratory shall ensure that it only uses suitable products and services when they are provided by external providers and have an influence on laboratory activities’. If the provider is not accredited, the laboratory must monitor the products and services to ensure their performance. In the case of an accredited provider, these controls can be greatly reduced. The accreditation of the service provider will allow the laboratory to take this competence into account and to consider it in its risk analysis.
Q: What was your perception of Bio-Rad as a company and more specifically the global quality management system?
The audit of Bio-Rad made it possible to realize that the level of quality requirements was already included in the structure. Admittedly, certain subtleties of the ISO 17025 standard were not considered, particularly with regard to the formalisation of the acceptance criteria for staff training, but on the whole, the rigor, traceability, and a concern for continuous improvement were already present. Bio-Rad obtained its accreditation (from its first initial audit, with little deviation) for the control of culture media according to the ISO 11133 standard, which requires a very large number of skills.
Question to Jean-Michel Plancq
Q: Why is quality so important to Bio-Rad?
Bio-Rad is a company that promotes strong customer relationships based on understanding and meeting their requirements, responding to their feedback, and providing support.
Q: How is the activity of the quality control laboratory organised?
The laboratory has built its own quality system which evolves within the existing system using a large part of the existing documentation. This integrates the new requirements of the standard and the documents issued by the French Accreditation Committee. In concrete terms, the laboratory has its own management review and internal non-conformity declaration process. The metrology, sampling, and results reporting procedures have been adapted and strengthened and operating procedures (or methods) have been developed.
Q: Can you explain how this new accreditation is complementary to the other ones?
As previously mentioned, the accreditation of the laboratory is fully integrated into the existing quality system and the specificities implemented are considered as strengths and not constraints. It allows us to be more efficient as well as ISO 13485 certification and compliance with GMP.
Q: You are used to accreditations for clinical diagnostics products, the ISO 17025 was the first for food and water testing products - what were the most challenging points for this implementation?
Standard 17025 has similarities with quality management standards such as 13485 and with GMP, however, some aspects were completely unknown to us. For example, the approach to the selection of methods and their validation, the rendering of results, or test reports. The use of the Cofrac logo makes us proud and also responsible because our approach is part of the continuity.
To conclude, accreditation of a suppliers' quality control laboratories increases confidence and facilitates the implementation of methods in user laboratories. This ISO 17025 approach is demanding and complementary to other supplier certification systems such as ISO 13485 or ISO 9001. ISO 17025 accreditation is now widely adopted by food and water microbiology laboratories. More recently, the application of this standard by the quality control laboratories of culture media suppliers has become a strong recommendation of some national accreditation bodies.
For more information on Bio-Rad’s complete range of food safety and water testing products, please use the green "Request Information" button below.