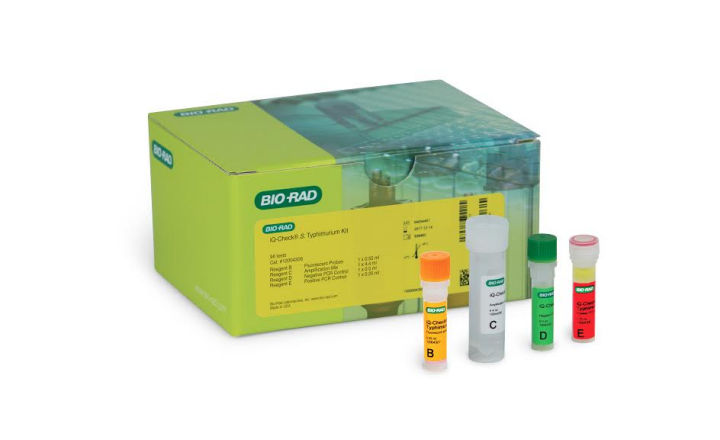
Salmonella spp. is the leading cause of food-borne diseases worldwide. Among its 2500 serovars, S. Typhimurium (ST) and S. Enteritidis (SE) are highlighted in many food safety programs, especially in the poultry industry. Bio-Rad has developed and validated two real-time PCR kits for the detection of these two key serogroups. Results of the validation study demonstrated the performance of the iQ-Check ST and SE methods to be equivalent to or better than the USDA and US FDA reference methods.
The iQ-Check S. Enteritidis and iQ-Check S. Typhimurium assays were approved for 25 g test portions of raw chicken breast with skin, raw chicken breast without skin, raw chicken breast without skin containing 2% w/w salt, raw chicken thigh with skin, raw chicken thigh without skin, boot swabs and drag swabs. The iQ-Check Free DNA Removal Solution was used for all matrices as a safe, non-toxic way to remove free DNA from the sample.
For more information on Bio-Rad’s complete range of iQ-Check real-time PCR test kits, visit www.bio-rad.com/iqcheck