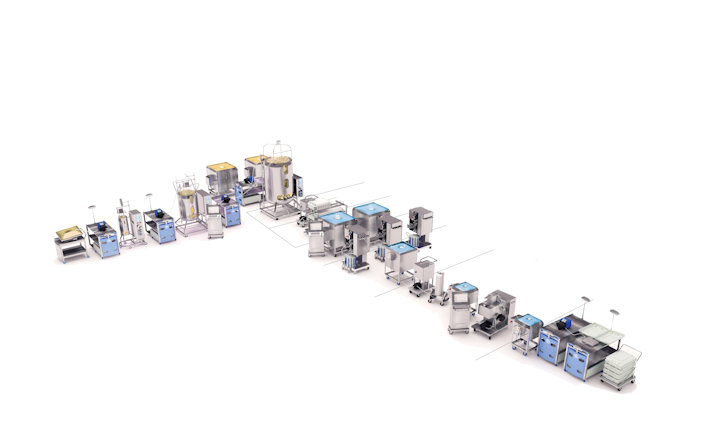
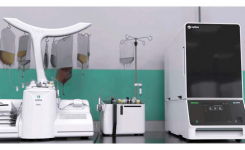
GE Healthcare's KUBio-"factory in a box" and FlexFactory platforms have enabled biopharma companies to quickly expand production of mAbs since 2012. In 2020, the KUBio portfolio is expanding to support the clinical and commercial manufacture of monoclonal antibodies, vaccines, and viral vectors for both cell and gene therapies. Michelle Stafford, Senior Global Product Marketing Leader, GE Healthcare LifeSciences, talks to rapidmicrobiology.com about how the KUBio solution fits perfectly to suit the requirements of biopharma companies needing to expand into these markets quickly.
Q: What issues do companies face when trying to increase production capacity?
A: The buy or build question is daunting when looking at production capacity planning. Both outsourcing production or manufacturing in-house involves dynamic analysis that includes timing, forecast, capability, geography and overall business strategy.
There needs to be enough product for clinical trials and any expanded use, which is identified during clinical trials as well as commercial needs. Supply must be addressed in due time, with proven and reliable high-quality biomanufacturing solutions.
Capacity capabilities need to rapidly scale up to meet requirements often on a tight timeline. Capex allocation will also be important whether spent on a reservation at a contract manufacturing organization or facility development. Depending on the unique business strategy, including the pipeline, IP protection, market introduction impact and long-term strategy, one option will be preferred.
The KUBio solution provides rapid and flexible production environments to enable capacity expansion. Compared to constructing a new facility, timelines are reduced, and this delays CapEx allocation and allows more time in clinical trials before commercial build decisions. With modular solutions, scale-up or scale-out can be accommodated through rapid expansion with modular facilities.
Q: How do the KUBio and FlexFactory address these problems?
A: Both KUBio and FlexFactory provide increased speed to market, adaptability to growth needs, and reliability on proven, safe, and dependable technologies.
GE Healthcare's KUBio is a prefabricated facility for the production of biopharmaceuticals equipped with a FlexFactory manufacturing platform and Figurate automation that can be tailored for the production of mAbs, viral vectors, and other modalities for biopharmaceuticals. A KUBio facility is constructed, assembled and fully fitted-out in 18 months, providing an accelerated timeline that is faster than traditional stick-built methods.
KUBio is a complete solution, which includes financial guidance, bioprocessing equipment, integrated Figurate automation, project coordination and qualification and a post-handover service package. Every stage of the project is supported by GE technical expertise, including facility design, procuring and production of process equipment, installation, qualification, staff training, technical support and project management from one point of contact. This facilitates the flexibility, speed, and quality needed by manufacturers of biopharmaceuticals.
Q: Can you give a real-life example where the KUBio module has expanded a company’s production, what are the typical applications?
A: To date, we have announced 4 KUBio facilities all in China. They are biosafety level 1 biomanufacturing facilities, which are typically used for mAbs.
Q: What are the logistics involved in moving KUBio and FlexFactory units overseas?
A: Modules and production equipment will be manufactured in various global locations. Delivery to the final location is coordinated by GE and may involve transportation alignment over multiple segments, including land and sea.
Q: What would be a typical time frame from project start to commissioning?
A: GE Healthcare's KUBio is a prefabricated facility for the production of biopharmaceuticals that are constructed, assembled and fully fitted-out, ready for handover after qualification in 18 months.
Q: What are the additional advantages of working with GE Healthcare, does using a KUBio facility reduce risk?
A: The KUBio solution is based on knowledge gained from the successful technical and operational deployment of over 60 Flex Factory single-use platforms and 4 KUBio facilities around the world. This package includes technical process optimisation, investment guidance, project management, automation integration, world-wide operational support, and single-use production platforms from a proven provider. Risk mitigation due to speed is part of the benefit of this more standardised modular solution as capital expenditures can be deferred until the success of clinical trials is more defined. Furthermore, risks of a fixed manufacturing workflow that can’t adapt to future needs are reduced with a flexible single-use manufacturing environment and the possibility to expand the modular facility.
Q: GE Healthcare has recently partnered with Pharmadule, what makes that a good combination?
A: With the growth of the biopharmaceutical industry, it is important to collaborate with industry specialists to develop additional solutions and help biopharma manufacturers access the market faster.
Pharmadule is an international company recognized for its excellence in constructing modular facilities, which, combined with our decades-long expertise in biomanufacturing, will help push the industry forward.
The speed and flexibility of the products in our KUBio offering is something which is well-recognised across the industry. With our know-how in the sector and global footprint, we are able to provide rapid deployment wherever a customer requires. Our KUBio offerings will continue to support the clinical and commercial manufacture of monoclonal antibodies, vaccines, and viral vectors (for both cell and gene therapies). We will also further expand our KUBio offerings, to include additional manufacturing scales as well as new product modalities.
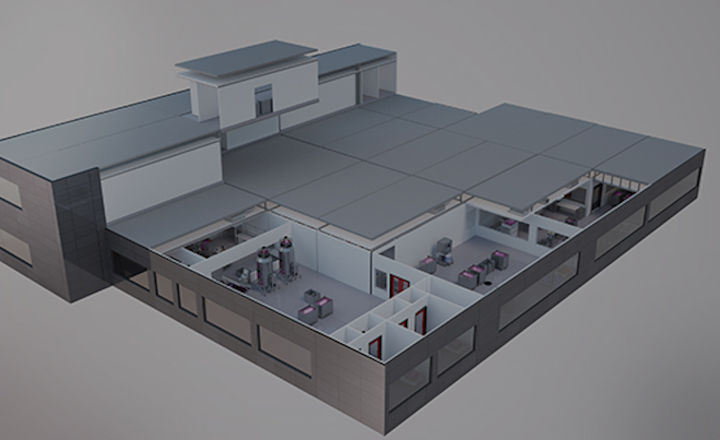
KUBio prefabricated facility equipped with FlexFactory platform. (Image courtesy of GE Healthcare)