*Product supplier listings (with product page links) provided at the end of the test method guide.
Key Points
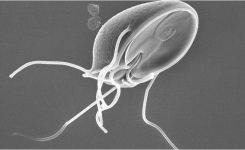
- Cryptosporidium parvum and Giardia intestinalis are water- and food-borne intestinal parasites, which pose a threat to human health in the developed world
- Standard detection methods for clinical and water samples are based on microscopy and require skilled technicians to perform them correctly.
- Diagnostic and immunological tests are commonly used in water treatment.
- Detection methods for the water industry require huge sample volumes and effective concentration procedures to detect the very low numbers of protozoan cysts likely to be present
- Newer immunology- and PCR-based detection methods are more sensitive, faster and easier to perform than microscopy and offer the possibility of differentiation of species and genotypes (Clinical)
Introduction: A wide range of intestinal parasites can be transmitted to humans directly, or via contaminated water and foods. Generally, these organisms are much more prevalent in developing countries with poor sanitation, but some are significant threats to public health in the developed world. The most important and widespread group of intestinal parasites are protozoa. Protozoa are microscopic single-celled organisms, some of which are parasites of animals, including humans. Several genera are capable of causing water- and food-borne illness, but the two most important in the developed world are Cryptosporidium and Giardia. Methods for the detection and identification of these protozoa have been developed for applications in clinical, water, and food microbiology laboratories.
Although there are many Cryptosporidium and Giardia species known (18 Cryptosporidium sp. have genetic data) methods used in water testing laboratories and clinical environments are to genus level, and so the exact species of parasite and hence its source is not known (or needed at the time).
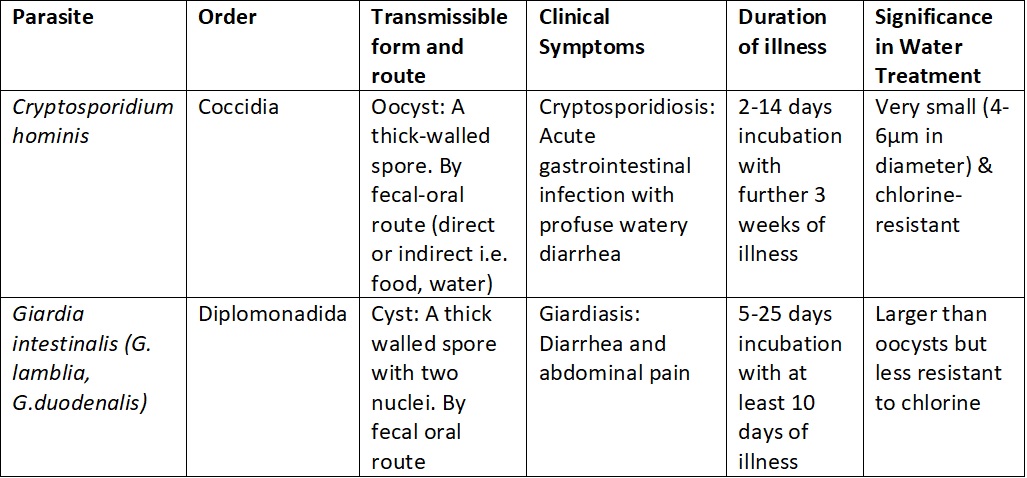
Table 1. Important information on the morphology and infectivity of the most prevalent parasites to infect humans.
Methods
Detection of the infective cysts of protozoan parasites cannot be accomplished by culture techniques and depends on sufficient cysts being present to detect by microscopy, immunological, or molecular methods. Methods for the detection of Cryptosporidium oocysts and Giardia cysts are very similar and can often be carried out simultaneously using the same assay. Most published and commercial assays have been developed for clinical and water applications, but methods for the detection of the parasites in certain foods are also available.
1. Water and environmental methods
International Standard Protocols: ISO 15553:2006 'Water Quality-Isolation and Identification of Cryptosporidium oocysts and Giardia cysts from water' is the only ISO method that applies to water testing for these parasites.
However, a UK public body, the Standing Committee of Analysts (SCA), publish a set of standard protocols, commonly called 'the blue books' and are the gold standard in water testing on the British Isles. The protocols are updated regularly and since last year have been made available for free online. As part of the series, the book 'The Microbiological quality of Water', Part 14- have a sectioned on these parasites; titled 'Methods for the isolation, identification, and enumeration of Cryptosporidium oocysts and Giardia cysts". This document has extensive detail and is complemented by a vast amount of microscopic images of both parasites, with different staining and contrast techniques applied. We have summarised main points from that document and the US EPA Method 1623.
Isolation by Filtration: Monitoring of treated drinking water, raw water, and wastewater samples for Cryptosporidium and Giardia cysts are hampered by the low numbers usually present. This means that large volumes of water need to be examined, especially when testing treated water. A concentration step is therefore essential, and current methods incorporate a filtration procedure before the detection method.
Collection and storage of samples in water treatment plants can be built-in so that large volumes (up to 1,000 litres for treated water) of water at different stages of the process can be diverted directly through a suitable filter. The filter is then sent to the laboratory for further analysis under refrigeration and processed as soon as possible. However, where remote testing is required, water can be collected in large carboys (10-50 litres) and transported to the laboratory chilled to a temperature below 10oC. Sample processing should begin within 96 hours of sampling.
Detection: The water sample is first passed through a filter system able to capture the small Cryptosporidium oocysts and Giardia cysts. Filter capsules designed specifically for the purpose are available commercially, and examples include the Envirochek™ capsule from Pall Life Sciences and the Filta-Max® system from IDEXX. The filter is then eluted, and the eluate centrifuged to concentrate the cysts, which are then separated from other material using an immunomagnetic separation (IMS) procedure. The IMS procedure can be semi-automated using the TCS Biosciences Isolate® system. The oocysts and cysts are then stained on well slides using labelled monoclonal antibodies and examined by immunofluorescence assay (FA) microscopy. Confirmation can be accomplished by staining with 4',6-diamidino-2-phenylindole (DAPI) and by differential interference contrast (DIC) microscopy. Staining systems for FA microscopy are available commercially and include the Meridian Biosciences MERIFLUOR system and BioPoint EasyStain™ system. The EasyStain has also been found to be suitable for use with PCR analysis. (Download report - Di Giovanni, G. D., R. M. Hoffman, and G. D. Sturbaum. 2010. Cryptosporidium Genotyping Method for Regulatory Microscope Slides, Project 4099 Report. Denver, CO: Water Research Foundation)
Molecular biology and PCR-based detection methods have also been developed for Cryptosporidium oocysts and Giardia cysts in water. As with clinical methods, these target specific sequences on the protozoan genome and can be used as alternatives to FA microscopy. Some commercial products are available, for example, CeeramTOOLS range from bioMérieux and Norgen Biotek market Cryptosporidium RT-PCR Detection Kits for water and environmental samples.
Identification: Unfortunately, the standard methods used in the water industry do not identify protozoan species or genotypes.
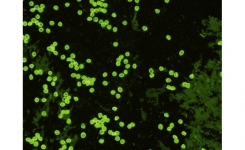
Quality Control and Reference Material: EasySeed™ is the global reference standard for Cryptosporidium and Giardia testing. It is approved by the UK Drinking Water Inspectorate and the US Environmental Protection Agency. ColorSeed™ is easy to use as an internal standard that can be added to every sample for Cryptosporidium and Giardia testing. The inactivated Cryptosporidium oocysts and Giardia cysts of ColorSeed fluoresce red, and those that were present in the sample fluoresce green.
2. Clinical methods
Diagnosis of both cryptosporidiosis and giardiasis can be accomplished by microscopic examination of duodenal aspirates and biopsy samples for sporozoites and other stages in the life cycles. However, the presence of large numbers of oocysts in the faeces allows detection by examination of stool samples, and most clinical methods are based on this approach.
Collection and storage of samples: Faecal samples for diagnosis of Cryptosporidium and Giardia infection should be tested as soon as possible, ideally within 24 hours. Alternatively, samples can be frozen or preserved with formalin and other fixatives. Since the shedding of cysts may be sporadic, at least three consecutive samples should be collected.
Detection Large numbers of cysts may be present in the faeces of infected individuals, and fresh samples can be examined without further processing. However, for samples preserved in fixatives, a concentration step may be necessary. Typically this involves a formalin-ethyl acetate concentration procedure with centrifugation to separate cysts from debris.
Microscopy: Direct microscopic examination of faecal smears prepared from fresh or concentrated samples is still widely used to detect protozoan cysts in faeces. As the cysts of Cryptosporidium, and to a lesser extent Giardia, are tiny, a staining procedure is required to identify them. A number of different staining techniques have been developed, but acid-fast procedures, such as the Ziehl-Neelsen stain and modified Kinyoun's stain, are commonly used, along with trichrome stain and fluorescence-based stains, such as auramine phenol. These methods require the skills of an experienced microscopist to be performed and interpreted correctly.
The current preferred method for the detection of protozoan parasite cysts by microscopy is based on immunology. Direct fluorescent-antibody (DFA) testing utilises fluorescent-labelled antibodies specific for cell wall antigens of Giardia cysts and Cryptosporidium oocysts to visualise the parasites and provide a definitive diagnosis. Commercial DFA tests are available, such as the widely used MERIFLUOR® Cryptosporidium/Giardia test from Meridian Biosciences, which are more sensitive than conventional staining techniques and easier to perform.
Immunology: Some immunology-based methods have also been developed to detect soluble protozoan antigens in faecal samples, thus removing the need for microscopic examination. These methods are now widely used in clinical laboratories for screening large numbers of samples quickly before confirmation by microscopy. Enzyme immunoassay (EIA) technologies have been used to develop commercial products for the detection of Cryptosporidium oocysts and Giardia cysts. These assays can be performed in a few hours using a microplate format, and the results read using a spectrophotometric plate reader. Examples include Remel's ProSpecT® microplate immunoassays and the RIDASCREEN® range of immunoassays from R-Biopharm.
Immunochromatographic lateral-flow 'dipstick' tests have also been developed for protozoan antigen detection in stool samples. These tests are sensitive and specific, but very easy to use and provide a result in 10 minutes. Examples of commercially available lateral-flow tests include the ImmunoCard STAT!® test from Meridian Biosciences and Remel's Xpect® test for Cryptosporidium.
Molecular biology: In recent years, molecular biology-based diagnostic detection methods have been developed for protozoan parasites. These are typically based on PCR or real-time PCR (RT-PCR) technology and target specific sequences in the protozoan genome. These methods offer highly specific and sensitive assays but have yet to be developed into widely available commercial clinical diagnostic products. However, the CeeramTOOLS range from bioMérieux includes molecular detection test kits based on RT-PCR technology that is marketed for both Cryptosporidium and Giardia in clinical samples and a few PCR-based tests for screening veterinary samples have become available.
Identification: Identification of Cryptosporidium and Giardia to species level is challenging by conventional methods and is a task for specialist parasitology laboratories. It is not usually necessary for routine diagnostic purposes but may be important in outbreak investigations. Recently, molecular biology techniques have made identification more practical, although specialist skills are still required. PCR followed by restriction fragment length polymorphism (RFLP) analysis or sequencing, targeting different parts of the genome, have been widely employed. Real-time PCR methods have also been described. For example, the Cryptosporidium Genotyping Kit from Invitrogen can distinguish between infective and non-infective species and genotypes.
3. Food methods
Food samples are rarely tested for Cryptosporidium oocysts or Giardia cysts. The low numbers likely to be present and the difficulty of extracting them from most food matrices preclude practical routine analysis. However, methods have been developed for certain 'high risk' foods, notably ready-to-eat salads and fresh produce. The FDA Bacteriological Analytical Manual (BAM) includes such a method designed to detect Cryptosporidium oocysts in fresh produce washes and filterable samples such as juices. The methods described are adapted from those used in water analysis and include both microscopy and PCR-based detection.
Get the latest updates in Rapid Microbiological Test Methods sent to your email? Subscribe to the free rapidmicrobiology eNewsletter