HardyCHROM™ CRE
Principle: Microbiology, Agar, Chromogenic Media
Automation: NO
Approvals: Company is licensed by the FDA and its quality management system is ISO 13485 certified.
Suitability: Chromogenic media, agar, microbiology, carbapenemase resistance screening
Capital equipment required: NO
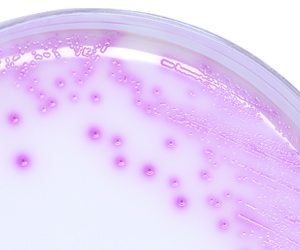
HardyCHROM™ CRE (Carbapenem-Resistant Enterobacteriaceae) is a selective and differential chromogenic agar medium intended for the qualitative and presumptive detection from stool specimens of Escherichia coli that are non-susceptible to carbapenems as pink colonies and KES (Klebsiella aerogenes, Klebsiella oxytoca, Klebsiella pneumoniae, Enterobacter cloacae complex, and Serratia marcescens) that are non-susceptible to carbapenems as blue colonies.
Key Points:
- Non-susceptible E. coli produces colonies that are rose to magenta in color with darker pink centers.
- Klebsiella, Enterobacter, and Serratia spp. produce large, dark blue colonies (with or without a pink halo), are presumptive positive for carbapenem non-susceptible KES (Klebsiella aerogenes, Klebsiella oxytoca, Klebsiella pneumoniae, Enterobacter cloacae complex, and Serratia marcescens).
- Colonies that are not pink to magenta, blue, or blue with pink halos are negative – No carbapenem non-susceptible Escherichia coli or KES detected.
Click here for more information
Areas:
Tags:
Hardy Diagnostics view full details
Santa Maria
United States
Website: Visit Website
Tel: +[1] 805-346-2766







