Legionella, A Waterb...
False Positive, or F...
1st July 2020 Content supplied by: R-Biopharm AG
Validated Immunoaffinity Techniques for Mycotoxin Analysis in Cannabis
The cannabis market has grown considerably over the last number of years following legalization for medicinal or recreational use in various countries. It has been estimated that in the US alone sales of cannabis products will exceed $2 billion over the next four years. As the sales of cannabis increases, so does the quantity produced worldwide. However, cannabis may contain toxic compounds such as pesticides, pathogens and mycotoxins.
Mycotoxins are secondary metabolites produced by moulds. The formation of mycotoxins depends on regional and seasonal environmental conditions, such as food availability, moisture content in substrate and surrounding air, temperature and pH value. Where conditions are right, fungi proliferate into colonies, and mycotoxin levels become high. Uncured or inadequately processed marijuana plant material could offer the right conditions for the growth of moulds.
Some mycotoxins are human carcinogens and as a result, are legislated throughout the world. Strict maximum levels are in place internationally for foods to ensure consumer safety. Following legislation of cannabis and cannabis products for either medicinal or recreational use in several countries, such products now need to be scrutinized for contaminants to the same extent as food or pharmaceuticals.
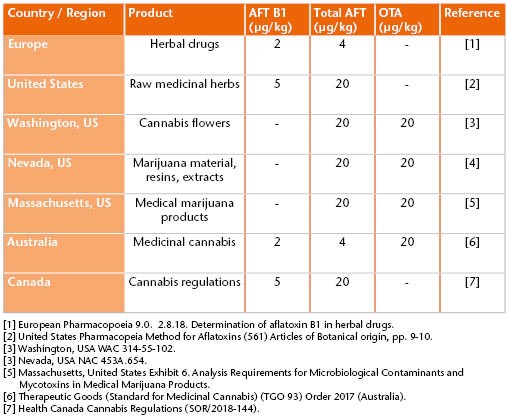
Therefore, mycotoxin analysis of cannabis and cannabis products is essential – but it can be challenging. Cannabis products will also contain other components than the analyte of interest, for example, terpenes, chlorophyll, oils, fats or sugars. All of these components can result in what is referred to as sample matrix, which can have a considerable effect on the quality of the results obtained and lead to issues in analysis.
It is, therefore, necessary to perform sample clean-up to prepare the samples for analysis. Immunoaffinity columns or cartridges are effective clean-up methods and are found to be highly specific as the mycotoxin of interest is isolated and concentrated from the sample. This makes the methods particularly suitable for the analysis of complex samples such as cannabis and cannabis products as immunoaffinity clean-up removes all interfering components from the sample giving you added confidence in your analysis which can ultimately help to reduce the number of samples re-analyzed.
In terms of mycotoxin analysis, immunoaffinity columns are already well established for the clean-up of a diverse range of complex matrices for all the regulated mycotoxins. These products have been rigorously validated and have been applied to a variety of botanical products such as herbal medicines, which have matrix similarities to cannabis.
In addition, immunoaffinity columns are typically used in official methods as they have been successfully used in various collaborative trials where it has been widely demonstrated that consistent, reliable results can be achieved. Immunoaffinity columns can be used for any sample type and offer particular benefits with complex or coloured samples such as cannabis as they are the only form of clean-up that completely removes all co-extractives from the sample resulting in clean eluates.
R-Biopharm has validated methods for immunoaffinity columns and automated cartridges for a wide range of cannabis and cannabis products (including flowers, capsules, tablets, oils, edibles and energy drinks) resulting in improved clean-up and concentration of toxins reducing chromatography interference, lowering detection limits and ensuring method performance criteria are met.
Further information:
- R-Biopharm solutions for mycotoxin analysis in cannabis (Link)
- Analysis of aflatoxin and ochratoxin A in cannabis and cannabis products by LC-fluorescence detection using clean-up with either multi-antibody immunoaffinity columns or an automated system with in-line re-usable immunoaffinity cartridges, Wilcox et al., 2019, Journal of AOAC International, jaoacint. 19-0176 (Link)
Tags:
Date Published: 1st July 2020
Source article link: View
Legionella, A Waterborne Risk
False Positive, or False Negative



